All published articles of this journal are available on ScienceDirect.
Factors Affecting Helminth Abundances in Synanthropic Rodents of an Urban Environment
Abstract
Background:
Current levels of urbanization cause changes in the ecology of hosts, the pathogens, or both, promoting the proliferation of zoonotic diseases. Rodents are a good biological model for the development of pathogen transmission models because it presence is often related to a none-adequate environmental management.
Objective:
The main goal of this paper was to study the changes in the abundance of helminth populations in synanthropic rodents of an urban landscape.
Methods:
A total of 92 R. norvegicus and 65 M. musculus were captured in the City of Buenos Aires (Argentina) and were screened for parasites. The variations in helminth abundances were studied at host population scale to determine the factors, such as the type of environment, meteorological conditions and demographic parameters of the hosts, which have an effect on helminth infection rates.
Results:
Parasites with intermediate hosts or free living larval stages in their life cycle were the most affected. It was found how rodents’ use of the habitats in the different urban environments has an effect on the helminth infection levels. Besides, the importance of season on helminth abundance was determined, suggesting that climatic conditions are crucial for parasite survival and transmission.
Conclusion:
This information is relevant because it not only allows us to deepen the ecological dynamics of parasites in urban rodents, but also shows that environmental conditions are determinants for the persistence of helminth populations in a city.
1. INTRODUCTION
The current levels of human–ecosystem interaction result in a habitat transformation and changes in the ecology of the host, the pathogen, or both, promoting thus an alteration in disease transmission dynamics [1, 2]. Urbanization is a phenomenon that accelerates and intensifies these impacts on zoonotic diseases [3, 4]. This is particularly observed for urban rodents because of the synanthropic behavior of these animals [5].
Mus musculus and Rattus norvegicus are among the best urban adapted rodents. Originated from Southeast Asia, various aspects of their biology, such as enormous reproductive potential, feeding benefited by accumulated or discarded human food, adaptations to urban environments, contribute to their success worldwide [6]. Synanthropic rodents are the main reservoirs or host for different human pathogens, including zoonotic helminth species [7]. The presence of murine rodents in anthropogenic habitats could be the reason for many of the parasitological studies performed worldwide [5], increasing the detection of rodent transmitted pathogens in last decades [8].
In the City of Buenos Aires (Argentina) (CBA), the community of rodents and their parasites has been studied for more than a decade. The establishment and proliferation of these animals are affected by the urban context, varying rodent composition according to habitat characteristics. Rattus norvegicus and M. musculus are the most common species and are the dominant species in two different landscape units of the CBA: shantytowns and parks or green spaces [9].
The helminth fauna of R. norvegicus and M. musculus of the CBA was recently described, exhibiting each rodent species its own characteristics in terms of richness, diversity and composition [10]. A high similarity was seen in the structure of the infracommunities of R. norvegicus from shantytowns and parklands, probably related to similar environmental characteristics that predict the presence of brown rats in both landscape units [11]. In contrasts, M. musculus is preferably captured inside the houses in shantytowns, exploiting thus microhabitats with different conditions regarding the specimens captured in parks, which result in quantitative differences between infracommunities from both landscape units [11]. However, the factors affecting the abundance of helminth populations of urban rodents are still unknown.
Parasites have been considered as excellent bioindicators of processes occurring in an ecosystem because their life cycles depend on the presence of intermediate hosts or are involved in trophic chains or its survival and development occur in specific environmental physical-chemical ranges, among many others [8, 12]. The greatest abundances of each parasite species are thus expected in sites where the resources (including the hosts) are in full and the conditions are propitious for the transmission and survival of free life stages [13]. The main goal of this paper is to study the changes of the abundance in helminth populations of R. norvegicus and M. musculus captured in the CBA and analyze the factors that explain these variations. It is expected that helminths with indirect life cycles were more affected by external factors than those with direct cycles and covariations are expected to occur between parasite species with similar life cycles and resource requirements.
2. MATERIALS AND METHODOLOGY
The study was carried out in Buenos Aires (34º37 'S; 58º24 'W), the main city of Argentina in terms of population and one of the largest metropolises in the world [14]. Information about the study area, rodent collection and parasitological prospection is provided in detail in Hancke and Suárez (2017) [10]. For the purpose of this paper, a total of 157 rodents, 92 R. norvegicus and 65 M. musculus, captured in 4 shantytowns and 3 parklands were analyzed.
First, possible variations in the abundance between landscape units were analyzed for both rodent species. Abundance was estimated by trap success and calculated as the proportion of traps with captured animals out of the total number of trap-nights for each site in each trapping survey. The relationship between trap success of R. norvegicus and trap success of M. musculus was analyzed for shantytowns, parks and totally by linear regressions. For each rodent species, trap successes between shantytowns and parklands were compared by analyses of variances (ANOVA). Trap success was root square transformed to meet normality assumption.
In parasitological studies, it is recommended to define the limits of parasite populations or communities at different scales. According to Bush et al. (1997) [15]:
Infracommunities refer to a community of infrapopulations, which include all individuals of a parasite species in a single host at a particular time. Component community refers to all infrapopulations of parasites associated with a subset of hosts in a particular time and place (or in a given ecosystem). Prevalence, mean intensity (I) and abundance (A) were calculated to describe the distribution of each parasite species in both host species following as recommended by Bush et al. (1997) [15].
For each host species, Permutational Multivariate Analyses of Variance (PERMANOVA) [16] were performed to study the effects of trapping season and landscape unit on the composition of infracommunities. To test if helminth species are independent of each others, the adjustment of the distribution of infracommunity richness frequencies to the model of Janovy et al., (1995) [17] was studied for both rodent species. This model assumes that, in the absence of associations and interactions among parasite species, the distribution of the observed frequencies of the infra-community richness can be predicted by the prevalence values of each of the parasite species. Therefore, each species would have an associated probability of infection which would be independent of the presence of other species in that host. All distributions were tested using the Chi-Square goodness of fit test.
Zero-Inflated Negative Binomial models (ZINB) were performed to study the relationship between the abundance of the helminth infrapopulations and the characteristics of the hosts [18]. Only the species whose prevalence values were equal or greater than 10% were included. ZI distributions can be viewed as a two-part model, in which the probability of species presence and abundance are modeled from the same data [19]. Particularly in Zero-Inflated Negative Binomial (ZINB) models, the probability of a zero outcome is modeled by logistic regression, while the continuous count is modeled by using a negative binomial error structure. The separate occurrence and abundance terms can represent different mechanisms due to factors operating at different temporal or spatial scales [19]. The number of worms per rat was considered as the dependent variable, whereas factors such as landscape units, season, sex, year, body length (covariable), abundance of R. norvergicus (covariable) and abundance of M. musculus (covariable) were tested in both the logistic and the negative binomial parts of the model as potential independent variables. The ZINB models were fitted manually, testing the significance of factors, covariable and interactions, first of the binomial part and then of the count part. The models were then progressively simplified by backward deletion and only significant terms (P < 0.05) were left in the final models. The models were selected with likelihood ratio tests and Akaike information criteria (AIC). For the particular cases of helminth species with low-intensity values, in this case, it makes more sense to evaluate the presence and not abundance, generalized linear models assuming a distribution of binary binomial errors or Bernoulli (presence / absence) with logit link function were conducted.
The relationship between the abundances of the most representative helminth species in each host species was tested in pairs with ZINB models. In each case, the abundance of one of the helminth species was considered as a dependent variable and the other as explanatory variable. As in the previous ZINB models, each host was considered asthe experimental unit. Given the small abundances of Hymenolepis nana and Hydatigera taeniaeformis, the presence instead of abundance was used as an explanatory variable. As the abundance of parasite can vary between environments, the landscape unit was included as a fixed factor.
All calculations were performed using the R version 2.15.1 [20]. PERMANOVA analysis was performed using the vegan package [21]. ZINB models were fitted with pscl package [22] and all likelihood ratio tests were carried out in lmtest package [23].
3. RESULTS
The abundances (or trap success) for R. norvegicus and M. musculus were independent of each other considering the total of the sites (slope=0.35, 95% CI [-0.72, 1.42], p>0.05, R2 = 0.02) as well as considering shantytowns only (slope=-0.94, 95% CI [-3.09, 1.21], p > 0.05, R2 = 0.08). For parklands, a positive relationship was observed between the trapping success of both rodent species (slope=1.43, 95% CI [0.44, 2.48], p<0.05, R2 = 0.50). While comparing abundance of the rodent, a higher trap success was detected in M. musculus in shantytowns (ANOVA, N = 25, F = 8.37, p <0.05) while no differences were observed for R. norvegicus (ANOVA, N = 25, F = 0.39, p > 0.05).
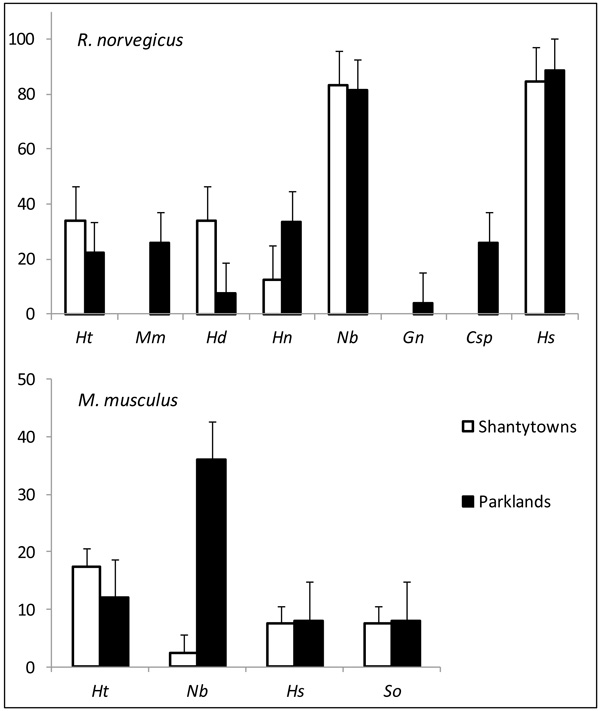
Rattus norvegicus was parasitized with a total of 8 helminth species (8 in parklands and 5 in shantytowns) while M. musculus harboured the same 4 species in both landscape units. Fig. (1) shows variations in the prevalence of these species between landscape units for both hosts. A more detailed information about the infection parameters is provided in Hancke and Suárez (2017) [11]. The PERMANOVA analysis revealed that the variations in the composition of infracommunities varied differently in R. norvegicus and in M. musculus Table 1. An interaction was observed between landscape units and the time of capture in brown rats. The effect of the time of capture was thus analyzed for each landscape unit separately and a significant effect was detected only for rats captured in parklands (shantytowns: PERMANOVA, Pseudo-F = 2.098, p> 0.05; parks: PERMANOVA, Pseudo-F = 2.971, p <0.05). For M. musculus, variations in the composition of infracommunities were detected between landscape units and between capture seasons Table 1.
| Factor | df | SS | MS. | Pseudo-F. | P | |
|---|---|---|---|---|---|---|
| R. norvegicus | ||||||
| Landscape | 1 | 0.30 | 0.30 | 1.21 | 0.297 | |
| Time | 1 | 0.40 | 0.40 | 1.62 | 0.163 | |
| Lansc x Time | 1 | 0.77 | 0.77 | 3.12 | 0.016 | |
| Residuals | 88 | 21.70 | 0.25 | |||
| Total | 91 | 23.17 | ||||
| M. musculus | ||||||
| Landscape | 1 | 0.29 | 0.29 | 2.22 | 0.057 | |
| Time | 1 | 0.54 | 0.54 | 4.20 | 0.001 | |
| Lansc x Time | 1 | 0.04 | 0.04 | 0.34 | 0.876 | |
| Residuals | 61 | 7.86 | 0.13 | |||
| Total | 64 | 8.73 | ||||
The distributions of the frequencies of species richness for both hosts did not differ from those expected by the Janovy model (R. norvegicus: χ 2 = 0,009; gl= 5; p= 0,999 (shantytowns), χ 2 = 0,334; gl= 8; p = 0,999 (parks); M. musculus: χ 2 = 0,007; gl= 4; p= 0,999 (shantytowns), χ 2 = 0,025; gl= 4; p = 0,999 (parks)). This means that the probability of infection by a single helminth species remains independent of the other species of an infracommunity. Table 2 summarizes the results of the generalized linear models for the factors affecting the helminth abundance. In the case of R. norvegicus, the type of landscape unit affected 3 species. On one hand, shantytowns had a positive effect on the presence of Hymenolepis diminuta and on the abundance of Nippostrongylus brasiliensis, while on the other side, parks had it on the presence of H. nana. Besides, the colder trapping season affected negatively the presence of H. diminuta and H. nana and negatively the abundance of H. spumosa. Both cestodes, H. diminuta and H. nana, were also affected by host gender and body length of R. norvegicus, while the abundance of N. brasiliensis and H. spumosa in rats were negatively and positively associated with the trap success of M. musculus respectively. In the case of M. musculus, the abundance of N. brasiliensis was positively affected by the colder trapping season and the trapping success of R. norvegicus and negatively with the abundance of M. musculus. Hydatigera taeniaeformis was analyzed for both rodent species, but no significant associations were found.
| Parasite | Variable | Predictor | Coeff. | Std. Err. | P | Effect | Life cycle |
|---|---|---|---|---|---|---|---|
| R. norvegicus | |||||||
| H. diminuta | presence | Shantytown | -1.674 | 0.864 | . | + | Indirect. Rodents as definitive. Arthropods as intermediate hosts. |
| Cold | 3.653 | 1.409 | ** | - | |||
| abundance | Male | 0.919 | 0.474 | . | + | ||
| H. nana | presence | Shantytown | -1.216 | 0.588 | * | - | Direct or Indirect. Arthropods as intermediate hosts. |
| Cold | -1.018 | 0.593 | . | - | |||
| Lengh | 0.022 | 0.013 | . | + | |||
| N. brasiliensis | abundance | Shantytown | 0.691 | 0.320 | * | + | Geohelminth |
| Ab. Mm | -0.086 | 0.0376 | * | - | |||
| H. spumosa | abundance | Cold | -0.621 | 0.315 | * | - | Direct |
| Ab. Mm | 0.072 | 0.040 | . | + | |||
| M. musculus | - | ||||||
| N. brasiliensis | Presence | Cold | 3.817 | 1.462 | ** | + | - |
| Ab. Mm | -0.608 | 0.187 | ** | - | - | ||
| Ab. Rn | 0.363 | 0.169 | * | + | - | ||
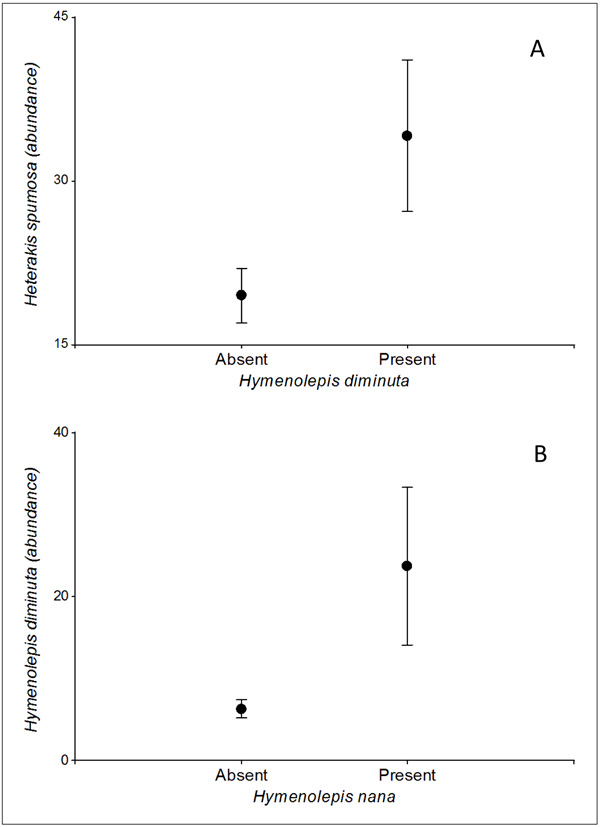
Regarding the interspecific helminth covariations, only significant effects were found on the abundance of H. diminuta. On one hand, the probability of infection of this cestode was positively associated with the abundance of H. spumosa (p <0.05), while on the other hand, its abundance increased with the presence of H. nana (p <0.05) (Fig. 2).
4. DISCUSSION
According to previous studies, helminth infracommunities of urban rodents could be grouped according to composition and relative abundances and they respond to the structure of the host community [11]. In this paper, we analyzed the variations in helminth abundances at a host population scale and identified the factors affecting the principal parasite species. Rodents are the main reservoirs or hosts of parasites relevant for public health. In this study, the presence of H. nana and H. diminuta confirms this role. Rodents represent a good biological model for the development of pathogen transmission models in urban landscapes because its presence is often a consequence of none-adequate environmental managements.
The type of urban landscape unit had a significant effect on the two intestinal cestodes found in our study. On one hand, a higher prevalence of H. diminuta was observed in shantytowns, showing that the conditions here favor the proliferation of arthropod pests as Tenebrio castaneum and T. confusum, both intermediate hosts of H. diminuta [24]. On the other hand, parks favor the presence of H. nana. This parasite is the most common cestode found in humans around the world, especially in children from poor areas [25, 26]. The parks sampled in our study area were located on the banks of rivers on whose shorelines precarious settlements not far from the parks take place. This situation would favor a greater organic matter contamination which could act as a source of intestinal parasite cysts or eggs, among other pathogens. However, despite both species were favored by different urban habitats at a landscape scale, a positive association between both species was observed. Hymenolepis nana is the only known cestode species that presents a direct cycle and occasionally uses arthropods as intermediate hosts, the same as H. diminuta. It could be hypothesized that both species may be responding at a microscale to similar biotic and abiotic factors, mediating also intermediate hosts the transmission of H. nana in R. norvegicus.
This is in concordance with the fact that both cestodes were also affected by the time of capture, decreasing both prevalences in the colder months. The development of intermediate host’s life cycle depends on optimal temperature and humidity conditions, affecting thus the abundance of the parasite [27]. Warmer months favor also the abundance of H. spumosa in R. norvegicus. Season is mentioned to affect the behavior of rats within urban environments, which could produce seasonal variations in the infection parameters of parasite populations [28]. The fact that H. diminuta and H. spumosa were both favored by warmer conditions could explain the positive relationship observed between both species. Besides, the time of capture also affected N. brasiliensis in M. musculus, but in this case, colder months favored the presence of this nematode. Seasonal variations in N. brasiliensis have been previously reported but for R. norvegicus captured in a shantytown of CBA [29]. As N. brasiliensis is a soil transmitted helminth and whose larval stages require adequate soil moisture conditions and are not resistant to desiccation, its surveillance in the environment is possibly conditioned in the warmer months of the year [30].
Host density and body length were also mentioned to be important factors that influence helminth infection rates, especially for directed transmitted parasite [31]. In our study, the infection likelihood of N. brailiensis in mice was favoured by greater densities of R. norvegicus. The abundance of this nematode was high in R. norvegicus (over 80%), so the infection of N. brailiensis in M. musculus is possibly related to the presence of brown rats, particularly in parks where the abundance of both rodents co-varied . Besides, the negative effect of mice abundance on the presence of N. brasiliensis is probably masking an effect of landscape unit more than an effect of population density itself, because a significant lower trap success for M. musculus was detected in parks.
Spatial variations in infection rates have already been described for a wide range of intestinal helminth communities for rodents in urban environments [12, 24, 32]. In our study area, while analyzing the variations of infracommunities in terms of composition, M. musculus exhibited differences between shantytowns and parklands. Mus musculus in both landscape units make use of different types of microhabitats because in shantytowns, it was trapped mainly inside the housings (in contrast to R. norvegicus who was captured mainly outside). For R. norvegicus, no differences were detected between the composition of infracommunities from shantytowns and parks, because the landscape unit did not affect the presence of the core species [10]. But an effect of the season was observed but only for rats captured in the parks. This suggests that seasonal fluctuations of the conditions for parasite survival and transmission may be reduced in the more human-dominated habitats like shantytowns.
CONCLUSION
To conclude, although host identity is a strong predictor of the structure of helminth infracommunities (diversity, composition, core species) within an urban rodent assembly [11], helminth infection rates are affected by external factors such as the type of environment, meteorological conditions and demographic parameters of the hosts. Particularly, parasites with intermediate hosts or free living larval stages in their life cycle are the most affected species. This information is relevant because it allows us to deepen the ecological dynamics of parasites in urban rodents and shows that environmental conditions are determinants for the persistence of urban helminth populations. The link between pathogens-rodent-environment is intimately connected, needing holistic approaches to address the prevention of zoonoses. Therefore, an improvement of the hygienic and environmental quality must be considered in any health program against urban zoonoses, particularly in shantytowns where social, economical and environmental conditions expose their inhabitants to a high risk of the so-called neglected diseases.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors wish to thank all the team of the Laboratorio de Ecología de Roedores Urbanos for their assistance during the field sampling. We are also grateful to Dra. Graciela Navone (CEPAVE-CONICET-La Plata-Argentina) for her help provided during helminth identification and her valuable comments on earlier versions of the manuscript. Financial support was provided by the University of Buenos Aires, Gobierno de la Ciudad de Buenos Aires and CONICET (Argentina).


