Amoebophagous Fungi as Predators and Parasites of Potentially Pathogenic Free-living Amoebae
Abstract
There are numerous case reports indicating that naked Free-Living Amoebae (FLA) can relatively easily get to humans or animals. The presence of pathogenic amoebae in habitats related to human activities supports the public health relevance of FLA. Acanthamoebae, Naegleria fowleri, Balamuthia mandrillaris and several other FLA have proved to be facultative human pathogenic microorganisms. Additionally, a wide range of FLA is known as vectors of pathogenic microorganisms (endocytobionts). Within their biocoenosis, FLA and fungi (and other microorganisms) live sympatrically. It is known that fungi serve as food sources for the phagocytotic active (mycophagous) trophozoite stages of FLA. In contrast, amoebophagous fungi may use FLA as prey organisms. Endoparasitic and predaceous fungi prove that there are numerous different interactions between FLA and fungi. The man-made introduction of suitable fungi into a habitat (soil or water) with human pathogenic FLA may overcome any ecological effects or limits. While nematophagous fungi have already been brought into action against harmful nematodes, the usage of amoebophagous fungi against FLA has not been widely considered. Nevertheless, the results from in vitro studies are promising concerning the targeted use of amoebophagous fungi as biological control measures against FLA in limited natural areas, in soil and in aquatic habitats.
1. INTRODUCTION
Naked Free-Living Amoebae (FLA) inhabit soil and aquatic habitats. Some of these FLA are able to form cysts as dormant stages resisting adverse environmental conditions. Within their biocoenoses, FLA are found sympatrically with other microorganisms. They live in biofilms of natural habitats and artificial man-made (engineered) habitats, grazing as motile, phagocytosis - active trophozoites on bacteria, algae and other microorganisms. As FLA and fungi can be found within the same environments, multiple interactions are to be expected. They may also feed on the hyphae, spores or conidia of fungi. The fungal stages are phagocytosed and lysed by the predatory, heterotrophic mycophagous amoebae. Throughout the years, however, a whole range of examples for microorganisms resisting this phagocytotic uptake was detected. Among those microorganisms surviving the uptake and escaping digestion, are numerous bacteria, viruses (especially the giant viruses) and also fungi [1]. This kind of interaction of FLA with other microorganisms is of particular importance with respect to Environmental Health. Those microorganisms surviving phagocytosis or even proliferating intracellularly, are termed endocytobionts. Various microorganisms, among them several fungi, have evolved strategies to survive phagocytosis by FLA and to exploit host cell resources. They even proliferate within their hosts, either intracytoplasmatic or endonuclear. There are quite a few examples for fungal endocytobionts.
On the other hand, FLA serve as prey for other predators, among them other protozoa or (non saprophytic) fungi. These amoebophagous fungi are abundant in soil, water, dung, bryophytes, decaying wood and leafmould. As predaceous and parasitic fungi, their abundance in a biocoenosis correlates with the abundance of their prey. As parasitic fungi, they may destroy their amoebal host following the uptake of fungal stages. The intracellular development of the fungi may enhance their virulence with consequences for animals and humans. Zoopagaceous fungi feeding on nematodes are studied to a certain extent while the amoebophagous fungi are not yet sufficiently examined to date with respect to their ecology.
2. INTERACTIONS OF NAKED FLA AND FUNGAL ENDOCYTOBIONTS WITH PUBLIC HEALTH RELEVANCE
The environmental basidomycetous fungus Cryptococcus neoformans is proved to proliferate within Acanthamoeba polyphaga and the trophozoite stages of Dictyostelium discoideum. As the amoebal host cells are lysed, the relationship must be called parasitic in this case. The virulence of the known human pathogen Cryptococcus neoformans has been demonstrated to be enhanced after its endocytobiontal phase within the host amoebae. Steenbergen [2] compared these interactions with the interaction of Cryptococcus neoformans and macrophages. Similar to these findings, Blastomyces dermatitidis, Sporothrix schenckii and Histoplasma capsulatum (order Onygenales) have been shown to reduce co-cultivated FLA populations [2, 3]. Penicillium spp. and Aspergillus spp. (a fungus causing several types of aspergillosis in humans) have been studied interacting with FLA: It has been shown that Aspergillus fumigatus conidia are capable of escaping from the food vacuoles and of germinating inside the cytoplasm of Acanthamoeba castellanii. The same mechanisms are used by Aspergillus fumigatus conidia to evade amoebal and macrophage digestive processes [4]. Aspergillus fumigatus conidia are also able to survive phagocytic processing by Dictyostelium discoideum [5, 6]. The incidence of Acanthamoeba and Fusarium species has increased in contact lens-related infectious keratitis, resulting in mixed infections. Studies on Acanthamoebae and Fusarium sharing the same environment revealed that the fungal conidia were internalized by Acanthamoeba castellanii strains and were able to germinate inside the amoebae [6-8].
3. AMOEBOPHAGOUS FUNGI: PARASITIC AND PREDACEOUS FUNGI
Similar to the nematophagous fungi, the amoebophagous fungi act, either as predators or parasites of their prey, the FLA. They are “(endo-) parasitic fungi” or “predaceous fungi” of FLA. Several amoebophagous fungi are obligate predators or parasites, some are facultative predators or parasites and are able to live saprophytically as well [9]. While there have been quite a range of morphological and phylogenetical publications of amoebophagous fungi in the recent years, the ecological impact and the applied scientific aspects have remained neglected (again in contrast to the nematophagous fungi) [10, 11].
3.1. Parasitic Fungi Predating FLA
3.1.1. Microsporidian - Like Fungi as Endoparasites of FLA
In “historical” publications of the last centuries, there are several hints of microorganisms developing in the nucleus of amoebae (Amoeba/Thecamoeba verrucosa). As early as 1895, the fungal genus Nucleophaga and the species Nucleophaga amoebae were described by Dangeard: “Thallus endobiotic, within the nucleus of the host, holocarpic; sporangium inoperculate, formed from the walled thallus, filling the nuclear cavity, spores simultaneously formed, set free upon the disintegration of the host body”. He concluded with: “A genus of uncertain relationships”. In 1902, Nucleophaga sp. was reported by Scherffel (1902) in Zygnema sp. (green algae; Fam. Zygnemataceae). Nucleophaga amoeboea was also mentioned by Penard in 1905 [12]. In addition, Doflein recognized in 1907, the formation of giant nuclei in Amoeba vespertilio, parasitized by an endonuclear fungus close to the Nucleophaga of Dangeard [13]. In 1916, Doflein stated that “Dangeard, Penard, Doflein, Chatton and Brodsky and others repeatedly found extraordinary nuclear parasites of amoebae leading to giant nuclei. They belong probably to the Chytridiaceae. These extraordinary parasites can also be found within the protoplasm of diverse Rhiziopoda (genus: Sphaerita)” [14]. In 1929, Doflein and Reichenow presented these Nucleophaga in their “Lehrbuch der Protozoenkunde”. They were found in two intestinal, apathogenic amoebae, Endolimax nana and Iodamoeba bütschlii. Subsequently, Nucleophaga was mentioned predominantly in the protozoological literature [15, 16]. Sparrow published in 1943 “Aquatic phycomycetes exclusive of the Saprolegniaceae and Phytium”, where he placed Nucleophaga within the Chitridiales. He further stated: “Since the vegetative body of the organism did not ingest the solid particles of the host, Dangeard considered Nucleophaga amoebae a chytrid allied to Sphaerita”. The activities of the host were not affected until sporulation of the parasitic microorganisms occurred. The host then disintegrated, allowing the spores to be dispersed. In 1949, Brumt provided descriptions of the Nucleophaga in his “Précis de Parasitologie” [19]. Parasitic intranuclear microorganisms, Nucleophaga hypertrophica, were responsible for the hypertrophy of the host nucleus (here: Intestinal amoebae). In the same year, the 6th Edition of the “Lehrbuch der Protozoenkunde” by F. Doflein, continued by E. Reichenow, was published, announcing a parasitism within Paramecium bursaria. The endoparasites were described as fungi, parasitizing in the cytoplasm (genus Sphaerita) or in the nucleus (Nucleophaga) of the protozoa. They use to grow either as multinuclear plasmodia, enveloped by a membrane disintegrating in multiple spores, or they show themselves as coccoid structures, proliferating by simple binary fission. In both cases, the outcome is the same: A regular bunch of coccoid structures is characteristic. The infestation with Nucleophaga leads to the formation of a giant nucleus, followed by an enlargement of the whole cell. Nucleophaga was found in Endolimax nana, a non - pathogenic intestinal amoeba. Reichenow wrote in 1949, that Brumpt and Lavier described a simultaneous infestation of Entamoeba dispar with Sphaerita and Endolimax nana with Nucleophaga. The Nucleophaga infestation led to the disintegration of the amoebal nucleus and the subsequent death of the cell.
Sphaerita, a fungal parasite within the cytoplasm of amoebae, was described by Dangeard in 1886. Similar parasitic fungi infecting protozoa were named Pseudosphaerita in 1894 [17]. Penard described Sphaerita endogena in 1905 [18]. He proposed the affiliation to the Chytridiaceae, and compared it with a parasite published later by Chatton and Brodsky in Amoeba limax [18]. In 1958, Sphaerita was detected in cysts of the apathgogenic intestinal Entamoeba coli [20]. Karling provided details about the “present status of Sphaerita, Pseudosphaerita, Morella and Nucleophaga” in 1972 [21]. Pseudosphaerita euglenae was described in 1995 as a parasite of Euglena spp [22].
The drawing of Nucleophaga amoebae in Amoeba is very similar to intranuclear microsporidia-like organisms (Scheid 2007), recently placed in the Rozellomycota [11] (Figs. 1 and 2).
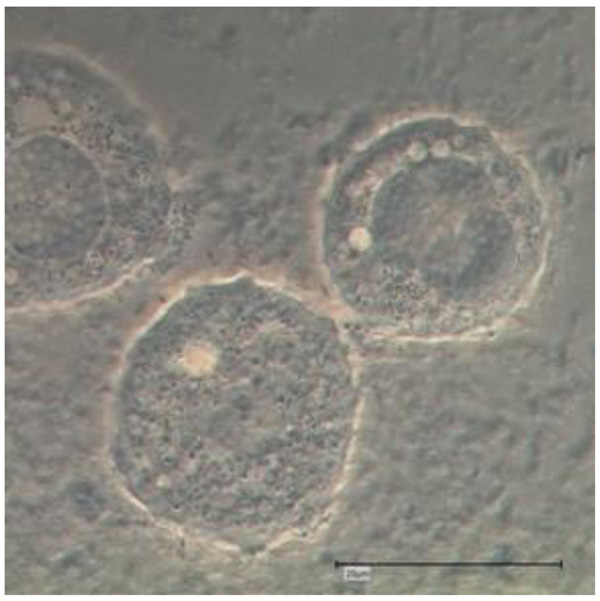
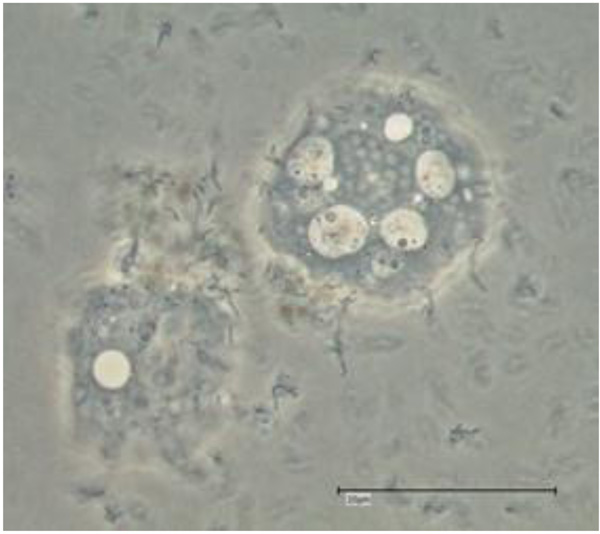
Whether these intracellular (and intranuclear) developing microsporidia - like endocytobionts are exactly these parasitic microorganisms Nucleophaga (or Sphaerita) described more than a hundred years ago, remains speculative to a certain extent: It appears that the Nucleophaga, described at that time, strongly resemble the members of the genus Rozellomycota. Electron microscopical photos were not available at that time confirming the affiliation. Details of the intranuclear parasites (and their hosts) were not sufficiently available and were not with an alternative (at least at certain stages): The described morphological findings resemble (at least in certain stages) the very recently described infestations of FLA with the so-called giant viruses (giruses). The drawings resemble (to a certain extend) some photo documentation of light microscopic examinations of Acanthamoeba polyphaga Mimivirus proliferating via their virus factories within FLA hosts (see and compare to the drawing of Nucleophaga by Sparrow in 1943 [17]). But in contrast, important detailed findings like the dilatation of the amoebal nuclear membrane and the amoebal plasma membrane points more likely to the development shown by Paramicrosporidium sp. (Rozellomycota; Paramicrosporidiales; Figs. 1 and 2). Notable details of another, similar, fungal intranuclear, parasitic endocytobiont in Tecamoebae were achieved and presented in 2010 using Calcofluor staining and fluorescence microscopy with a filter of 355-425nm [23, 24]. This intranuclear endocytobiont belonged to the Rozellomycota and was called (the rediscovered) Nucleophaga amoebae [25].
3.1.2. Parasitic Fungi Developing Intracellularly
Cochlonema sp. was initially described by Dechsler as an (endo-) parasite of naked FLA. Cochlonema pumilum and Cochlonema fusisporum (infesting testaceous rhizopods) were detected in 1939 whereas Cochlonema euryblastum and Cochlonema bactrosprum var. longius were described by Drechsler in 1942 [26, 27]. Cochlonema euryblastum (order Zoopagales; Figs. 3 and 4) lives on FLA trophozoites. In 1946, Drechsler revealed details of Cochlonema agamum [28]. As most of the descriptions of Cochlonema and other fungal endocytobionts date from that time, presumed new descriptions of detected intracellular parasitic fungi should (also) initially be carefully compared morphologically with photos, drawings or depictions in those publications.
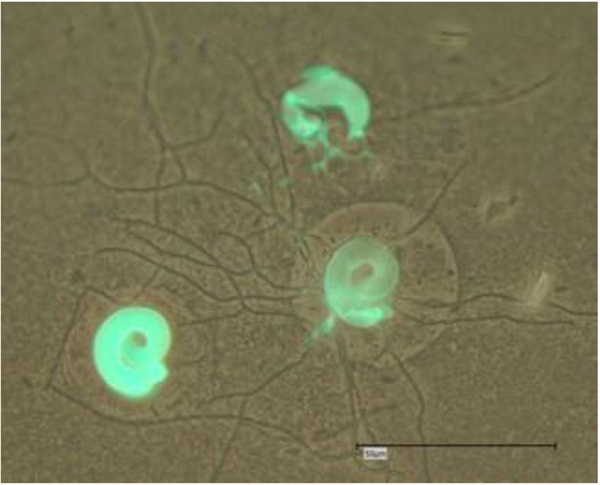
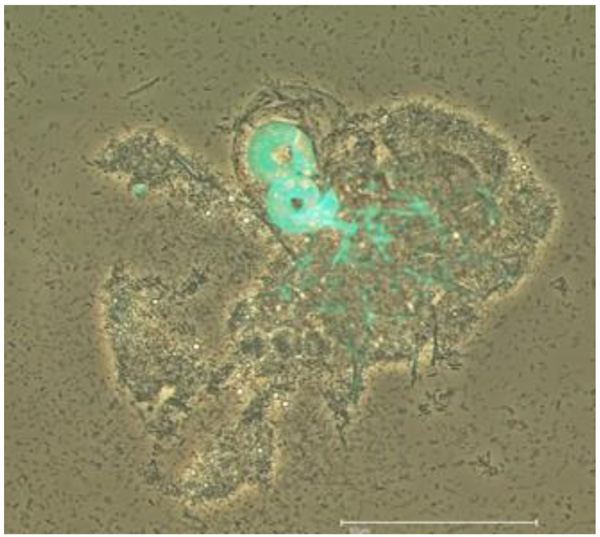
Saikawa and Sato provided the ultrastructure of Cochlonema odontosperma, an endoparasite in amoebae [29]. 18S rDNA sequencing was used for the molecular identification and phylogenetic affiliation of Cochlonema euryblastum to the zoopagales in 2007 [30]. In 2010, the germination and morphology of the zygospores of Cochlonema cerasphorum (originally described by Drechsler 1959 [31]) and Cochlonema megalosomum (described by Drechsler in 1939 [32]) were studied [33]. Morphological details and developmental stages of Cochlonema euryblastum were published by Kurek et al. 2010 [24]. The morphological details of Cochlonema euryblastum while parasitizing Thecamoeba quadrilineata trophozoites were presented using Calcofluor White staining and fluorescence microscopy: The thalli and hyphae (and also the conidia) of Cochlonema growing in amoebal trophozoites could be colored by Calcofluor White staining due to a high chitin amount (Figs. 3 and 4). Subsequently, the combination of (stapled) photos with phase contrast and those with fluorescence staining revealed an impressive documentation of the morphological details and the developmental stages [24].
The development starts with the uptake of the conidia by the FLA trophozoites (ingestion of the conidia by the FLA). Cochlonema sp. turns out to be endoparasitic. The fungal conidia are not digested but they germinate, producing coiled hyphae (thalli), penetrating the amoebal cell wall. The first stages germinate vesicularly at the end of a conidium inside the amoebal cytoplasm. Following a growing phase, the thalli transform into spiral forms, still inside the cytoplasm. Cochlonema produces large thalli inside the host amoeba followed by a penetration of the amoebal cell wall [34]. With the fragmentation of the hyphae in conidia and the uptake of these conidia via FLA trophozoites the cycle restarts, whereas more than one conidium may germinate inside an amoeba.
3.2. Predaceous Fungi Predating FLA
Several predaceous fungi capture FLA trophozoites by exploiting specific trapping structures. They come in contact with their prey, the FLA, by adhesive hyphae [34]. Adhesion at the “sticky” hyphae, invasion of the trophozoites and production of “haustoria” are the essential steps of predation. Genus and species of these predaceous fungi were usually determined by the morphology of the conidia, whereas their morphology depends on the environmental conditions [35]. As early as 1911, the fungal genus Zoophagus was described by Sommerstorff as a haustorial parasite or predator of rotifers [17]. Acaulopage spp. and Stylopage spp. (e.g. Stylopage araea), both orders belonging to the Zygomycetes, Zoopagales, are well described as predaceous fungi capable of lysing FLA.
Charles Drechsler studied Stylopage sp. (Stylopage rhabdospora and Stylopage cephalote) from the 1930s. In 1935, he described Stylopage araea, Stylopage haploe and Stylopage lepte as predacious fungi of amoebae [36]. In 1938, Stylopage cephalote was published as being a parasite of amoebae [37]. In 1939, the description of “five new Zoopagaceae destructive to rhizopodes and nematodes” followed, among them the amoebophagous Stylopage scoliospora (a predacious mycelial form rather than an infective or parasitic fungus) and Stylopage rhynchospora [26]. In 1946, Drechsler described Stylopage rhabdospora and Stylopage rhicnacra in detail and provided excellent drawings [28, 38]. Stylopage cymosa was found in dung by Duddington in 1953 [39]. The same scientist studied Stylopage haploe and Stylopage rhabdoides in detail and provided drawings of the haustoria, the conidia and zygospores [40]. He stated, that “amoebae that came into contact with the mycelium were held, apparently by adhesion, and an outgrowth from the hypha at the point of contact penetrated into the endoplasm of the animal and branches repeatedly to form a system of haustorial filaments”. In 1955, Stylopage araea var. Magna was described by Peach and Juniper in comparison to the same species described by Drechsler in 1935 [36, 41]. Another three predacious fungi (Cystopage sphaerospora, Cystopage ellisospora and Stylopage rhabdospora) were investigated by Dayal and Srivastva in 1979, still providing drawings of the morphological features [42]. Islamov described a new species of amoebophagous fungi and named it Stylopage apsheronica [43]. The first description of Stylopage anomala as a new species (from dung) followed in 1983 by Wood [44]. The ultrastructure of Stylopage rhabdospora as an amoeba - destroying fungus was reported in 1986 [45]. 1991 followed with a detailed description and a study of the dispersal of Stylopage anomala, addressing the question of arthropod dispersal (phoretic mites) [46].
Acaulopage tetraceros was described by Drechsler from soil [36]. Drechsler seems to have detected Acaulopage tetraceros as amoebophagous fungus, capturing Thecamoeba sp. He described several species of Acaulopage and provided excellent morphological details of haustoria, conidia and hyphae, only to mention Acaulopage macrospora and Acaulopage ceratospora in 1935, Acaulopage cerospora in 1936, Acaulopage stenospora in 1941, Acaulopage lasiospora and Acaulophage gomphoglada, in 1942 and Acaulopage ischnospora in 1947 [27, 36, 47, 48]. In 1946, Drechsler provided details of Acaulopage lophospora and Acaulopage hystricospora [28]. In 1948, he reported on findings concerning host specificity among the amoebophagous fungi such as Acaulopage baculispora, and he described Acaulopage gyrinodes [38]. Peach worked on Acaulopage dichotoma in Britain from 1948 and on aquatic predaceous fungi [49, 50]. Ingold and Ellis recorded spores of Acaulopage tetraceros from water [51]. Jones and Peach described a variety (or a pleomorphical variant) of Acaulopage tetraceros, which showed that the fungus traps trophozoites before it sporulates [52, 53]. Park (1971) examined the function of the tubular extension of the conidium and observed the germination of these conidia [53]. Among the genus Acaulopage with its 27 species, all of them are associated with the predation of FLA (type species Acaulopage rhaphidospora) [54, 55], among them Acaulopage gomphoglada, Acaulopage dichotoma, Acaulopage tetraceros and Acaulopage lasiospora. Studies on the amoebophagous fungi Acaulopage dichitoma and Acaulopage tetraceros were conducted by Saikawa and Kadowaki in 2002 [35]. In Acaulopage dichotoma, they found zygospores for the first time. They confirmed that Acaulopage tetraceros and Acaulopage dichotoma also captured amoebae with their adhesive hyphae in aquatic environments [36]. The germination and morphology of zygospores of Acaulopage laphospora (described by Drechsler in 1946 [28]) were studied in detail in 2010 [33]. In 2015, the prey pattern, cultivation methods and molecular characterization of Acaulopage tetraceros were published [56].
The predaceous fungi can easily be detected and cultured as they can be recognized by the caught amoebal trophozoites adhering to their fertile environmental hyphae [34]. Co-cultivation assays revealed prey pattern, host spectrum and fungus-FLA interactions, which consist in three main phases: The process starts with the adhesion and immobilization of amoebal trophozoites at the hyphae of the fungus (adhesive phase). The predaceous fungi show capture structures with terminal adhesive, sticky points.
The predaceous fungi such as Acaulopage sp. or Stylopage sp. penetrate the amoebal cell wall and invade the cytoplasm of the trophozoites producing narrow finger- or bush-like branching haustoria (Fig. 5) as intruding feeding hyphae (invasive phase) [56]. Multiple trophozoites of cyst forming and non - cyst forming FLA can be captured simultaneously with one hypha and multiple haustoria (lytic phase; lysis). Mycotoxins seem to play a major role in killing the captured amoebae [58]. The amoebophagous fungi seem to not host specific regarding their prey pattern, while not every prey amoeba tested in co-culture assays stimulated conidia formation [56, 59-62]. In addition to the naked free-living amoebae, Dictyostelium sp. and Fusarium sp. (both slime molds) were captured while they were forming amoeboid stages [56]. Prey preferences in nature while meeting mixed prey amoeba populations seem to occur, depending on biochemical factors (such as lectins, surface proteins etc.). The conidia production is dependent on various environmental factors. Cyst-forming FLA try to escape invasion of the fungal hyphae by encystation, as the cysts are more resistant against the intruding fungi, which are usually not able to penetrate directly through the cyst wall. It could be demonstrated in vitro, that in certain cases, the haustoria of Stylopage sp. and Acaulopage sp. may also intrude into cysts of FLA, whereby the haustoria penetrate through the cyst pores, e.g. of Willaertia magna [60, 61] and Naegleria gruberi, respectively [59].
CONCLUSION
Environmental and climatic changes leading to a global warming may have an influence on the FLA abundance, which may, in turn, lead to an increase of infectious human diseases associated with FLA or their endocytobionts. The diversity of fungi and protozoa (including FLA) in the environment (especially in soils) and their complex interactions are currently under investigation [63].
Several fungi use other microorganisms as a nutrition source. Nematophagous and entomophagous fungi have been studied for their suitability as an alternative, biological method of parasite control. This approach results from the applied sciences within the field of biological control and has led to a wider examination of the taxonomic affiliation. Beauveria bassiana has been tested for many years against the phytoparasitic bark beetles (e.g. Ips typographus). Metarhizium anisopliae has been shown to act as entomophagous fungi against sheep scab mites (Psoroptes ovis) and cattle lice (Bovicola bovis). The well described and studied nematophagous fungi use mycelial structures to capture free-living nematodes or to parasitize worm eggs. They may harm the helminths as endoparasites (initially infecting nematodes with their spores), predators (trapping with adhesive cells and a constricting ring) or opportunistic fungi (while parasitizing eggs and female nematodes). Duddingtonia flagrans is an example of such a nematophagous fungus colonizing feces and killing the developing helminth larvae. A whole range of nematophagous fungi has been studied and applied as biological control organisms against nematodes, which are harmful to plants, crops or humans (e.g. geohelminths).
Recent studies on amoebophagous fungi focus on their morphology or their phylogenetic affiliation rather than their ecological role or their practical use against FLA with a public health significance. Amoebophagous fungi may be used in soil or aquatic habitats against pathogenic FLA in a similar way as the nematophagous fungi are used against plant pest nematodes or human pathogenic nematodes. These amoebophagous fungi could contribute to our ability to avoid or significantly reduce FLA populations, colonizing e.g. thermal bathing ponds, where humans can contact the FLA. As a result, during in vitro studies with a limited space of the Petri dishes with FLA and nutritional bacteria, especially the predaceous fungi were able to extinguish the whole FLA population of the used strain. Particularly, the non- cyst forming FLA were caught and lysed subsequently to a full extent.
Another aspect of their potential use to protect public health is the fact that amoebous fungi may act in soil or water against FLA found on grown vegetables. Besides the likelihood of contact with pathogenic FLA, these FLA may harbor pathogenic bacterial food - associated microorganisms such as Staphylococci, Campylobacter sp. or Listeriae. For example, it is difficult to get alfalfa sprouts or cress sprouts free from pathogenic microorganisms, as disinfection measures may not always be successful. Study results reveal that protozoa such as Tetrahymena sp. (a cilate), Bodo saltans (a flagellate), Cercomonadae (flagellates) and FLA (Acanthamoeba spp. and Vannella spp.) have been detected on sprout tissues [64]. The direct impact of such foodborne FLA as pathogens or vectors remains undetermined. The amoebophagous fungi in soil or water may have an impact also in this scope of application.
Therefore, the amoebophagous fungi should be considered as a biological control tool of potentially pathogenic FLA, particularly in cases in which the niche of the FLA is environmental, limited and well - known. Although widespread, the natural distribution and presence of amoebophagous fungi in a given habitat is dependent on the habitat conditions, and not exclusively on the presence of prey amoebae [10]. The man-made introduction of suitable fungi into a habitat (soil or water) of human pathogenic FLA for biological control may overcome the ecological effect. The ambient temperature is important with respect to the successful use of amoebophagous fungi as active predators. The optimum-temperature and the preference temperature respectively may be determined in vitro before the targeted use in natural warm areas. Whether ubiquitous or specialized species are useful for this purpose has yet to be determined.
An example of such potential use could be the use of amoebophagous fungi against pathogenic (thermophilic) naked FLA in restricted habitats, e.g. when cohabitating in a source of natural thermal water. Another example is the targeted use of amoebophagous fungi in artificial lagoons or isolated/restricted soil habitats, in which Balamuthia mandrillaris has been detected in several cases [65]. Furthermore, thermal recreational waters could be “treated” with obligate amoebophagous fungi to serve as predators or parasites against Naegleria fowleri. It appears that the contamination of water with Naegleria fowleri (the “brain eating amoebae”) occurs in these cases after the organism emerges from geothermal sources when the water runs over the soil. Thus, the soil around these sites is responsible for the contamination of the natural thermal baths [66].
A selective and well-aimed application of predaceous or parasitic fungi seems to be expedient and promising. The spores and conidia of Acaulopage tetraceros could be used after cultivation and drying as a spore/conidia/nutrition substrate powder because of the high tenacity [61]. The confirmed wide host range potentially enables Acaulopage tetraceros to be used to reduce or control several pathogenic FLA.
The reduction of those FLA acting as hosts of pathogenic endocytobionts (e.g. legionellae) provides an additional, indirect benefit. The usage of amoebous fungi in soil and water is possible as for example Acaulopage tetraceros has been isolated from water sediments as well. Similar approaches concerning the use in aquatic habitats (such as water pipes) may apply for Stylopage araea. These obligate amoebophagous fungi might be capable of keeping a certain areal free of FLA, which is especially important when these defined areals, e.g. soil around hot (thermal) springs, are related to human activity.
To date, there are no existing studies of the targeted application of amoebophagous fungi in natural risk areas with confirmed pathogenic FLA abundances. The monitoring of interactions with FLA results exclusively from in vitro studies. Possibly, the combination of two or more biological control agents (e.g. predaceous fungus and parasitic fungus simultaneously) could enhance their activity to decimate the relevant FLA or at least reduce them below a “damage threshold”. However, a combination of two or more amoebophagous fungi could result in incompatibility or antagonism. The real effectivity of amoebophagous fungi with respect to the reduction of natural FLA populations in natural habitats needs verification by means of field studies. But it is certainly worth considering since such a biological control method could provide long-term and sustained effects, is non- toxic, and would not be harmful to humans or pets.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author would like to thank Dr. David Lam (MD, MPH, Shaman Medical Consulting) for review and English language editing of the article.


