Antineoplastic Chemotherapy and its Effects on the Gastrointestinal Parasitism of the Dog
Abstract
Background:
In veterinary medicine, an increasing incidence of neoplastic diseases has been followed by a growth in the use of chemotherapeutic drugs, often associated with opportunistic infections.
Objective:
This study aimed to assess the prevalence of gastrointestinal parasites in dogs undergoing antineoplastic chemotherapy in the Lisbon Metropolitan Area, as well as their evolution throughout the protocol and respective effects of chemotherapy on dogs’ lifestyle.
Methods:
Faecal samples were collected in a group of 30 dogs being treated for neoplastic diseases under different protocols, previous (G1) and during chemotherapy (G2). In total, 60 samples were analysed by Willis flotation, natural sedimentation, Baermann technique and faecal smear stained with Ziehl-Neelsen. A survey to characterize animal lifestyles and deworming care were also conducted with dog’s tutors.
Results:
In total, there were two positive samples for the protozoan Giardia sp., one of which is in association with the nematode Toxascaris leonina. The two dogs only obtained positive results during chemotherapy (G2). An overall prevalence of gastrointestinal parasitic diseases of 6.7%, in G2, and 0%, in G1, was obtained.
Conclusion:
The low parasite prevalence has not allowed the evaluation of an association between the use of antineoplastic compounds and infections by gastrointestinal parasites. However, it was concluded that the studied groups were efficiently dewormed, as well as they did not experience any obvious changes in their parasitological component and its lifestyle.
1. INTRODUCTION
In contemporary society, the relationship between man and dog, as a companion animal, has become increasingly strong, and the dog is often considered as an integral element of the family. Despite advances in treatment and prophylaxis of parasitic diseases, parasites can cause high rates of dog morbidity and mortality, as well as public health implications, given the zoonotic potential of some species [1]. Among the most affected canine groups, young, geriatric and/or immunocompromised animals are the most represented [2]. In the latter group, dogs are included with neoplastic disease under chemotherapy process.
In veterinary medicine, the increasing incidence of neoplastic diseases has been accompanied by an increase in the use of chemotherapeutic agents often associated with opportunistic infections [3]. Based on the studies of human medicine that indicate an increased risk of opportunistic gastrointestinal parasitic diseases in patients undergoing chemotherapy, the authors decide to explore this idea applied to dogs as oncological patients.
Thereby, this study aimed to assess the prevalence of gastrointestinal parasites in dogs submitted to chemotherapy, the evolution of parasitism throughout the protocol, and the characterization of associated risk factors for infection.
2. MATERIALS AND METHODS
2.1. Sample Composition
Sixty faecal samples were collected from 30 dogs under different standard chemotherapeutic protocols. Two samples per canid were collected at different times analysed before initiating the chemotherapy protocol (G1) and during the protocol (G2).
For the inclusion of cases, there was no distinction between sex, age, breed or types of neoplasias. The time gaps between the two sample collection considered the pre-patent periods of the dog’s most frequent gastrointestinal parasites and were preferably greater than one month [4, 5]. Dogs that died during treatment, or whose faecal collection was not possible to achieve, or those who had to interrupt the chemotherapeutic protocols for unrelated reasons were excluded. In addition, surveys were carried out to tutors to characterize the lifestyle of their dogs and the deworming practices. The clinical histories of each animal and the blood counts performed at the times of faecal collection were also considered.
This study was approved by the Faculty of Veterinary Medicine, University of Lisbon, Ethics and Animal Welfare Committee and informed owner consent was obtained prior to study enrolment.
2.2. Study Area
The 30 canids sampled were resident in the Lisbon Metropolitan Area. The samples were collected at the Veterinary Hospital of Restelo and at the Small Animal Hospital, Faculty of Veterinary Medicine, University of Lisbon, from November 2016 to September 2017.
2.3. Collection and Management of Faecal Samples
The mean interval of days between the collection of G1 and G2 was greater than one month, with an average of 59 days. Each faecal sample consisted of around 5 grams of fresh, day-old stool collected at home by tutors or, on the day of the chemotherapeutic session, by the work team, into a sterile plastic container. After collection, the samples were stored at 4ºC and transported to the laboratory until 48 hours later, for analysis.
2.4. Parasitological Diagnosis
Macroscopic examination of faecal samples for identification of adult parasite forms, mucus or blood, were followed by microscopic screening. For the laboratory analysis, Willis flotation, natural sedimentation technique, Baermann technique and faecal smear stained by the modified Ziehl-Neelsen technique were performed [6-8].
2.5. Tutor Survey
A survey was performed to each tutor of the canids sampled. It was conducted as an oral personal interview, focusing mainly on the lifestyle, walking habits and deworming practices of each dog sampled. The survey format was a multiple-choice based interview that took approximately 2 minutes to fill. The questions were formulated in order to be simple, objective and easy to understand.
2.6. Statistical Analysis
The results of coprological analyses, tutor surveys and clinical histories of the animals were stored in a Microsoft Excel 2007® spreadsheet and later imported and analysed by R program, version 3.4.3. (2017), using the R Commander extension.
3. RESULTS
3.1. Laboratory Results
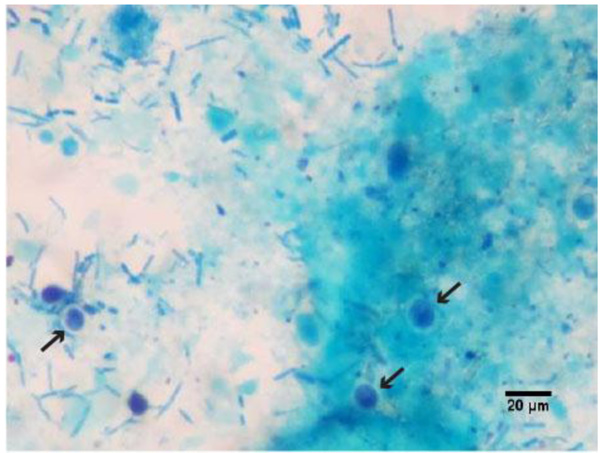
Of the surveyed dogs, 6.7% (2/30) were positive for one or more species of intestinal parasites at the moment of the collection during the protocol (G2); the first samples analysed, before the protocol has started (G1), were negative in both animals, 0% (0/30). An association of Toxascaris leonina (Fig. 1) with Giardia sp. (Fig. 2) was found in one sample, 3.3% (1/30), while the other presented a solo infection by Giardia sp., 3.3% (1/30) (Table 1).

| Variable | Percentage (No.) of Positive Responses | |
|---|---|---|
| G1 (30) | G2 (30) | |
| Specie | – | – |
| Giardia sp. | 0% (0) | 6.7% (2) |
| Toxascaris leonina | 0% (0) | 3.3% (1) |
| Positivity | 0% (0) | 6.7% (2) |
| Negativity | 100% (30) | 93.3% (28) |
3.2. Sample Characterization
The whole sample consisted in adult animals (over 1 year old (y.o.))., with an average age of 10 y.o. About 53.3% (16/30) of the dogs were males while 46.7% (14/30) were females. Approximately 60% of the dogs were purebred (Boxer, Cocker Spaniel, Labrador Retriever, Rafeiro do Alentejo and Rottweiller were the most represented breeds). The two positive dogs were Cocker Spaniel males with 12 y.o.
3.3. Pet Management
The majority of the dogs, 80% (24/30), lived in a domestic environment with tutors and the remaining 20% (6/30) in kennels. Every dog was fed with commercial dry food and/ or homemade diet (30/30). Raw meat was not given to any animal. About 70% (21/30) of the dogs lived mainly indoor and 60% (18/30) cohabit with other animals (Table 2).
| Variable | Percentage (No.) of Positive Responses | |
|---|---|---|
| Total (30) | Positive Animals (2) | |
| Residency | – | – |
| Domestic | 80% (24) | 50% (1) |
| Kennel | 20% (6) | 50% (1) |
| Lifestyle | – | – |
| Indoor | 70% (21) | 100% (2) |
| Outdoor | 30% (9) | 0% (0) |
| Diet | – | – |
| Commercial dry food | 63.3% (19) | 50% (1) |
| Homemade diet | 6.7% (2) | 0% (0) |
| Raw feeding | 0% (0) | 0% (0) |
| Combination of commercial dry food and homemade diet | 30% (9) | 50% (1) |
| Cohabitation with animals | – | – |
| Dogs | 46.7% (14) | 50% (1) |
| Cats | 20% (6) | 0% (0) |
Around 83.3% (25/30) of the tutors walked their dogs in public spaces, like streets and parks, 68% (17/25) daily, 20% (5/25) at least once a week and 12% (3/25) less than 1-3 times a month. During the walk, about 76% (19/25) had frequent contact with other dogs (Table 3).
| Variable | Percentage (No.) of Positive Responses | |
|---|---|---|
| Total (25) | Positive Animals (2) | |
| Walking frequency | – | – |
| Daily | 68% (17) | 50% (1) |
| Once a week | 20% (5) | 0% (0) |
| Less than 1-3 times a month | 12% (3) | 50% (1) |
| Walking location | – | – |
| Urban places (i.e. streets) | 28% (7) | 50% (1) |
| Green places (i.e. parks, gardens, beaches) | 28% (7) | 0% (0) |
| Urban + Green places | 44% (11) | 50% (1) |
| Contact with other dogs | – | – |
| Frequent | 76% (19) | 50% (1) |
| Rare | 24% (6) | 50% (1) |
| Never | 0% (0) | 0% (0) |
3.4. Parasite Control Practices
About 43.3% (13/30) of the analysed canids already had external parasites, at least once in their life, while 3.3% (1/30) had internal and external parasites.
At the time of the study, out of the total number of dogs surveyed, 70% (21/30) were considered well treated with endoparasitic drugs, according to ESCCAP guidelines (ESCCAP, 2018a). The most commonly used anthelmintic (AH) drugs were the combination of praziquantel-pyrantel-febantel with an expression of 52.4% (11/21), followed by the combination of praziquantel-fenbendazole, with an expression of 23.8% (5/21). Compounds including macrocyclic lactones were used in 19% (4/21) of the time. Deworming practices were performed in parallel with the recommended treatment, at least four times a year, by 57.1% (12/21) of the tutors, 28.6% (6/21) dewormed three times a year, 4.8% (1/21) twice a year and 9.5% (2/21) once a year (Table 4). The majority of the tutors with more than one dog at home, 71.4% (10/14), dewormed them with endoparasiticides; all the tutors that practiced deworming in their sick dog also dewormed every other healthy dogs and cats at home.
| Variable | Percentage (No.) of Positive Responses | |
|---|---|---|
| Total (21) | Positive Animals Dewormed (1) | |
| Internal parasites | – | – |
| Four times a year | 57.1% (12) | 100% (1) |
| Three times a year | 28.6% (6) | 0% (0) |
| Twice a year | 4.8% (1) | 0% (0) |
| Once a year | 9.5% (2) | 0% (0) |
Regarding the control practices with ectoparasiticides, 76.7% (23/30) of the tutors treated their pets regularly. Fluralaner (oral formulation) was the most used compound with 47.9% (11/23), followed by imidaclopride-permethrin (spot-on combination) with 34.8% (8/23). Half of the pets dewormed with spot-on formulations used as well deltamethrin collars with 17.4% (4/23). The frequency of treatment varied mainly between four times a year, 56.5% (13/23), and every month, 26.1% (6/23), depending on the ectoparasiticide used. There were even tutors who only treated their pets seasonally, 8.7% (2/23), or once a year, 8.7% (2/23). The tutors with more than one dog at home treated them all equally.
3.5. Neoplastic Disease and Associated Treatment
There were represented 13 different neoplastic diseases, including multicentric lymphoma, 33.3% (10/30), followed by mast cell tumour, 16.7% (5/30), and osteosarcoma, 10% (3/30). For each neoplastic disease there were different therapeutic protocols, including conventional, metronomic and targeted chemotherapy. The dogs with positive samples were subjected to different models of chemotherapy: One was treated for lymphoma with a conventional protocol denominated CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin and prednisolone); the other was treated for anal sac carcinoma with a targeted protocol using tyrosine kinase inhibitors like toceranib phosphate.
3.6. Clinical Parameters
At the time of the two collections, different clinical parameters including clinical signs, blood count and antibiotic therapy were analysed.
The clinical signs varied between anorexia, pasty stools, diarrhoea, vomit and cough and their frequency is represented on Table 5. In general, there was a greater expression of clinical signs in G2 (33.3% (10/30), when compared to G1 (20% (6/30). Three dogs presented clinical signs in both moments.
| Variable | Percentage (No.) of Positive Responses | |||
|---|---|---|---|---|
| Total (30) | Positive Animals (2) | |||
| Clinical signs | G1 | G2 | G1 | G2 |
| Anorexia | 6.7 (2) | 10% (3) | 0% (0) | 0% (0) |
| Soft stools | 6.7% (2) | 6.7% (2) | 50% (1) | 0% (0) |
| Diarrhoea | 0% (0) | 10% (3) | 0% (0) | 50% (1) |
| Vomit | 10% (3) | 3.3% (1) | 50% (1) | 0% (0) |
| Cough | 3.3% (1) | 6.7% (2) | 0% (0) | 0% (0) |
Regarding blood counts and referring to G1, 6.7% (2/30) of the dogs presented neutropenia, one of them with an associated lymphopenia. There was one dog, 3.3% (1/30), who had eosinophilia. In G2, 10% (3/30) of the patients had some type of cytopenia: one with neutropenia, other with neutropenia and lymphopenia associated and another with monocytopenia and eosinopenia. The two dogs with positive faecal samples did not present any change at blood counts.
About antibiotic therapeutic, it was evaluated if the dogs were submitted to the metronidazole therapeutic until 2 weeks before the sample collection. There were two dogs performing antibiotic therapeutic only at the time during chemotherapy; none of them had positive laboratory results.
4. DISCUSSION
During the present research, only two dogs showed positive faecal samples, both in group G2, during the chemotherapy protocol. One dog showed infection by Giardia sp. (3.3%) and another a mixed infection by Giardia sp. and Toxascaris leonina (3.3%).
Taking into account the parasitological results, Giardia sp., most probably Giardia duodenalis was the most prevalent parasite in this study, which is not surprising given that it is one of the most common gastrointestinal parasites in domestic animals worldwide [9]. Recent studies in Portugal have demonstrated the prevalence of Giardia sp. in kennels from approximately 20 to 60% of the animals [10-13]. In Lisbon, a prevalence of 11.4% was observed in faecal samples collected from dog parks [14]. Concerning Toxascaris leonina, prevalence of 1 to 6% were obtained in kennels from Portugal [13, 15, 16]. In Lisbon, 1.1% of faecal samples from canine parks were positive for T. leonina [14]. The fact that it is an ascarid present in the soil of the parks of the Lisbon Metropolitan Area constitutes an important risk factor in parasitic infection, due to their high resistance in the environment to external abiotic factors.
Concerning the tutor survey, it was possible to analyse some risk factors that, similarly to healthy dogs, could predispose to parasitic infection. Regarding housing, about 20% of dogs living in kennel constituted a higher risk population, since a study carried out in kennels from Portugal indicated that 25% of the analysed kennel dogs had an active parasite infection [15]. About the type of feeding, no animal was fed with raw food, a risk factor for acquiring parasitic infections, by the ingestion of infected hosts. In addition, the environment in which they lived was mainly indoor and this could lead to less access to sources of infection, compared to animals living in an outdoor environment.
The frequency and walking habits of dogs undergoing chemotherapy did not differ substantially from healthy animal studies in the same region [17, 18]. Most of the dogs in the present study had access to the street and about 76% of these had frequent contact with dogs with unknown parasite history. The parasitological status of these animals being unknown may constitute a risk of transmission for parasitic infections. Matos et al. [17] reported that green places, compared to urban places, are the ones where tutors are less likely to collect faeces from their dogs. Also, Smith et al. [9] observed a positive association between the prevalence of gastrointestinal parasites, mostly protozoa, and the use of public parks by dogs. The majority of the dogs in this study walked into green places like public parks, contributing to an increased risk of parasitic transmission.
Concerning parasite control practices, taking into account the anti-parasitic drugs used and the frequency of deworming, 70% of the tutors were considered to have regular prevention with endoparasiticides to their dogs. Compared to other studies in the Lisbon region, this is a lower value [17-19]. This difference may be due to the fact, that no animals in this study with irregular antiparasitic treatments were considered dewormed and some surveys were incomplete due to the lack of knowledge of the tutors. On the other hand, the canids analysed in this research corresponded to a global population older than the other studies, with an average of 10 y.o. According to the study of Matos et al. [17], the old canines were the group of animals dewormed less frequently or not dewormed at all. Another study reported that animals older than 10 years of age were the most affected by endoparasite infections, after the group of animals less than 6 months of age [20]. This may be explained by a greater weakness of the immune response in geriatric animals or because tutors do not maintain regular deworming practices by mistakenly assuming that canids become resistant to parasitic infections throughout life.
Regarding the endoparasiticides used, the associations of praziquantel-pyrantel-febantel and praziquantel-fenbendazole were the most frequent in this study, agreeing with previous studies [17-19]. Both are broad-spectrum associations for gastrointestinal helminths and may be administered, with a good safety margin, in the dog sample involved. Concerning the frequency of deworming, the most common frequencies were every 3 or 4 months. When compared to the studies in healthy dogs whose most common frequency was every 6 months, the results of the present study are closer to the frequency recommended by the ESCCAP guidelines [4, 5], which is every 3 months. This evidences that the tutors in this study, when deworming their pets, are more conscientious regarding frequency, compared to tutors of healthy animals.
For each animal, clinical signs and white cell blood counts were also evaluated at the time of the faecal sampling. This evaluation was made independently of the type of protocol performed given the low prevalence of metronomic protocols in this study. Regarding the clinical signs, there were evaluated predominantly gastrointestinal signs mostly related to some type of internal parasitic diseases and to chemotherapy side effects. Overall, at the time of the second sample collection there appears to have been a greater expression of clinical signs compared to the first samples. Diarrhoea and soft stools were the most frequent clinical signs in dogs undergoing chemotherapy, followed by anorexia, and these were found to be in about 1 in 4 animals analysed, according to results described in the literature [21].
Regarding the association with endoparasites, only one of the two parasitized dogs showed clinical signs at the time of the second collection, corresponding to diarrhoea. In this animal, diarrhoea could be justified by being parasitized with Giardia sp. and Toxascaris leonina. The remaining animals that showed clinical signs or no diagnosed parasitic infections, the signs could be due to the gastrointestinal toxicity associated with the chemotherapeutic agents or to the neoplasia itself.
The white blood cells count was evaluated in an attempt to assess the state of the immune system of each dog at the time of each sample collection. Several studies showed alterations in white blood cells count in dogs undergoing chemotherapy, including cellular populations as neutrophils and eosinophils [22, 23]. Despite of these studies, it was not possible to take conclusions in the present research considering that none of the animals with low counts at the time of sample collection had positive parasitological diagnoses. None of the animals also presented eosinophilia, often associated with helminth infections [24].
In the course of this research, no studies were found to correlate the effects of antineoplastic chemotherapy on the gastrointestinal parasitism of dogs, for which a similar bibliography on human medicine was consulted. In these studies, human patients undergoing chemotherapy were evaluated and there were large variations in the prevalence of gastrointestinal parasites, with values between 10 and 90% [25-32]. Protozoal infections were observed in all studies, with particular emphasis on Giardia duodenalis and Cryptosporidium spp., which were detected in most studies. Helminth infections were also frequent, mainly attributed to nematodes belonging to the families Ascarididae, Ancylostomatidae or Trichuridae. In the present study, Giardia sp. and a species of nematode belonging to the family Ascarididae, Toxascaris leonina, were identified and therefore the groups of parasites found in the present research were in agreement with the bibliography of human medicine. The low prevalence observed, compared to studies in humans, is probably due to the deworming practices, which are a more common habit in veterinary medicine when compared to the human one. Other factors such as the animal lifestyle, with greater access to possible sources of infection, may have influence on parasite prevalence. Only one of the studies cited above described the deworming practices performed in human patients: Silva et al. [31] reported that only five of the 18 subjects analysed human patients in their study performed antiparasitic treatments prior to the start of chemotherapy, corresponding to 27.8% of the study sample. When compared to the prevalence of 70% of dewormed canids regularly in this research, the values are expressively different, corroborating the previous considerations. It is also important to note that studies in human medicine have been carried out in developing countries and / or mostly in tropical climates, especially in South America and Eurasia, which could predispose a high prevalence of parasitic infections, in comparison to developed countries with temperate climate, as in the case of Portugal.
Regarding the techniques used, false negatives should be considered, especially when diagnostic techniques with different sensitivities are used. In addition, the fact that some of the samples were not analysed in the same day as they were collected constituted a limitation of this study. On the other hand, the absence of parasitic elements in the great majority of the sample can express a real absence of parasitic infection or presence of an infection with low parasitic load, not detected with the techniques used. Moreover, in some parasites, such as Giardia spp., the release of cysts is intermittent, which makes it difficult to diagnose them, resulting in lower prevalence than the actual one [5]. In this study, given the unavailability of the tutors and / or the clinical condition of the sampled animals, only one-day faecal sample was collected. The prescription of three coprological exams on three consecutive days should considerably increase the sensitivity of these techniques [5].
The low overall parasite prevalence observed in the present study, compared to studies in healthy canines, may be due to two factors. The tutors of these dogs being a highly concerned population with veterinary care and / or all of the canids sampled were a population of older dogs, with parasite load, egg excretion and less active infections, compared to young dog populations, as evaluated in other studies [17-19].
Finally, analysing the two positive animals in detail, the two presented several differences. The chemotherapy protocols were a conventional and a metronomic, reinforcing the fact that it is important to carry out this type of researches in the two modes of chemotherapy. Regarding endoparasites, only one canine presented concomitant history of internal and external parasitism and a positive coprological result during the study. The fact that this result was obtained only in the second collection, corresponding to the moment of chemotherapy, indicates that, despite having a history with previous endoparasites, the positive result in the second collection may correspond to a new parasitic infection, acquired during the time since the beginning of the chemotherapy protocol. Relative to the similarities between these two canids, both were geriatric animals and therefore, with the weakened immune system, functioning as an important risk factor. In addition, both were of the Cocker Spaniel breed, highly predisposed to acute adverse effects associated with chemotherapy, such as gastrointestinal signs and myelosuppression, according to Couto [33], something that would be interesting to investigate further.
CONCLUSION
The low parasite prevalence of the first and second sample collections, showing only Giardia sp. and Toxocara leonina infections, did not allow an association between the use of chemotherapeutic agents and infections by gastrointestinal parasites. Prevention with antiparasitics proved to be efficient in this group of dog tutors, although the group of dogs sampled represented a group of animals that were more susceptible at the immune level, either by the chemotherapeutic protocol to which they were subjected or by the neoplastic disease itself. Finally, through the results of the tutor surveys, the present sample of canines, despite their clinical condition, presented, in general, lifestyles and walking habits similar to a population of healthy canines, with small differences.
It is important that more studies can be performed on this subject, in order to obtain a broader and more incisive knowledge, since according to the literature this is the first research regarding this subject involving dog-oncological patients. It would be interesting to obtain larger samples, to use more sensitive diagnostic techniques, taking into account the parasites found in this research, to study more carefully the most frequent chemotherapeutic protocols and to do the additional research of other parasitic forms, namely intracellular parasites such as Leishmania infantum or Toxoplasma gondii.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All clinical, collection and laboratory procedures performed were in accordance with the ethical standards of Portuguese College of Veterinary Surgeons and the Ethics and Welfare Committee (CEBEA) of the Faculty of Veterinary Medicine, University of Lisbon.
HUMAN AND ANIMAL RIGHTS
No humans/animals were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Informed consent was obtained from all the tutors of the dogs in this research while filling the survey formulary.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACNKOWLEDGEMENTS
This research was conducted as a part of the Master Thesis of João Lory Costa as the Faculty of Veterinary Medicine, University of Lisbon (FMV-ULisboa), Portugal [34]. The authors would like to thank to the Centre for Interdisciplinary Research in Animal Health (CIISA), Faculty of Veterinary Medicine, University of Lisbon, as well as to the Veterinary Hospital of Restelo and the Small Animal Hospital, Faculty of Veterinary Medicine, University of Lisbon, and to the tutors who kindly cooperated with this study. Project UID/CVT/276/2013 (CIISA) funded this research.


