All published articles of this journal are available on ScienceDirect.
Co-infection Paragonimiasis-pulmonary Tuberculosis Discovered from a Man in Abidjan, Côte d’Ivoire: Case Report
Abstract
Introduction:
Paragonimiasis is a very uncommon zoonosis in sub-Saharan Africa. It is a trematodial disease contracted by eating fresh or undercooked crustaceans harboring a parasite of the genus Paragonimus. About fifteen species of Paragonimus are pathogenic to humans. In Africa, three species are frequently encountered: Paragonimus africanus, Paragonimus uterobilatéralis, and Paragonimus westermani. Clinically, it has similarities with pulmonary tuberculosis so that confusion is quickly made.
Case Report:
We report here a case of paragonimiasis discovered incidentally in a security guard man, 26 years old, originated from Côte d’Ivoire, who consulted in 2019 to the Medical Teaching Hospital of Cocody, for hemoptysis under a chronical mode.
Results:
After examination, the disease of paragonimiasis was diagnosed by positive direct smear. Otherwise, patient was positive to TB after a molecular test.
An anti-tuberculosis cure was proposed, and a treatment with praziquantel 600 mg (2 tabs x3 / d for 4 days) allowed us to obtain biological cure without major side effects for paragonimiasis.
Conclusion:
The diagnostic issues of paragonimiasis constitute a great challenge for health systems already weakened by the instability due to covid-19 pandemic. The insufficient resources allocated to the health sector showing the lack of integration of the diagnostics of paragonimiasis to pulmonary tuberculosis program are crucial.
The strengthening of human resources, as well as the improvement of the technical platform of reference laboratories in regions, are really needed.
1. INTRODUCTION
Paragonimiasis is an anthropo-zoonosis represents a real health problem in the world. It is estimated 293.8 million people are at risk of paragonimiasis disease and 20.4 million people infected [1, 2].
Humans are infected by consuming fresh or undercooked crustaceans, which themselves harbor the metacercariae of Paragonimus sp. Among dozens of species found in vertebrates, only 8 are present in humans and can cause disease [3].
This disease is rife mainly the Asian continent because of their risk eating habits.
In Africa, nearly twenty countries are affected by pulmonary distomatosis. The majority of cases have been carried out, mainly in Nigeria and Cameroon [4].
Cote dIvoire is the third country in terms of frequency. Indeed, the first case was discovered in 1947 from a woman of 36 years-old after lobectomy by Bertrand et al. [5]. After this case, Coulibaly et al. detected a case of co-infection Schistosomiasis-Paragonimiasis from a child, originated from the west of the country [6].
Subsequently, five cases reported by Assalé in 1977 in the southwest of the country [7]. Then, Bossé worked in this field and reported nine other cases in the same region [8].
Another case was discovered in 1988 in the north of Côte d’Ivoire by Adoubryn et al. [9].
Over the past 20 years, most work has been carried out by Aka et al. in the south, southwest, and west of the country, respectively in Lauzoua’s island, Divo, Lakota, and Abidjan. These studies allowed us to assess the knowledge, attitude, and practice of practioners, as well as the identification of some intermediary hosts of Paragonimus [10-13].
We report here a case of pulmonary paragonimiasis discovered fortuitously in a patient.
2. CASE REPORT
This case concerned Sir D.B, 26 years old security guard, originated from Côte d’Ivoire, resident in Abidjan. The medical history started 3 months ago. The patient was victim of recurrent cough in January 2019. This cough was described as banal because it was temporary. However, at the beginning of April, the patient presented with the same cough, this time associated with chest and retrosternal burn-like pain triggered by the effort of coughing. Low abundance hemoptysis, without sign of retention, progressing in a context of night sweats, without fever or weight loss had been reported. There were no dyspnea, asthenia,. Consultation a health center lead to the initiation of antitussive and antibiotic treatment, which not eliminate the symptoms. The patient, therefore, opted for self-medication based on traditional products (plants), which without satisfaction.
The patient report the consumption of shellfish cooked in sauce. A notion of regular alcohol intake is admitted with no precision of the frequency.
While on duty, the patient, on April 14, 2019, with hemoptysis of around 100 cc with dizziness, which led him to seek urgent medical attention at the Teaching Hospital of Cocody.
2.1. On Admission
Vital signs: T = 101.8 ° F, Weight = 70 kg, Height = 1.77 m, Pulse = 62 bpm, Blood pressure: 115/75 mmHg, SO2 = 99% Breathing rate = 17 cycles/mn
Good general condition, colored conjunctiva, clean tongue, no dehydration, no edema of the lower limbs, normal consciousness (Glasgow: 15)
Clinical examination was poor apart from the crackling, basi-thoracic rales found on examination of the pleuro-pulmonary system.
In the course, the patient carried out biological check-ups, as shown in Table 1.
| Nature of Analysis | Date | Results |
|---|---|---|
| Hematology | 05/02/2019 | • Cytobacteriologic examination of bronchial fluid - Négative • Hemogram: - Hb: 12,5 g/dl ; - WC: 4.4x103/mm3 - RC: 5.26x106/mm3 - PNN: 79%, PNB: 0%, PNE: 02%, Monocytes:3% Lymphocytes: 16% • Blood Group and Rhesus status: AB Positive • CRP:48 (vr:<6 mg/l) • Hemogram: - Hb: 13,8 g/dl ; - WC: 5.97x103/mm3 - RC: 5.65x106/mm3 - PNN: 69%, PNB: 0.3%, PNE: 7.9%, Monocytes:6.7% Lymphocytes: 16.2% • CRP: 192 mg/l • TP: 76% (vr>65%) • TCA:41 sec (vr:32 sec) • TQ:15 sec (vr:13.05 sec) |
| - | 04/24/2019 | |
| 04/22/2019 | ||
| 04/15/2019 | ||
| 04/14/2019 | ||
| Parasitology | 29/04/2019 | • Direct examniation of Sputum - Positive (Paragonimus sp.) |
| Bacteriology | 04/29/2019 | • Xpert MTB/RIF - MTB detected, sensible to Rifampicin • Smear microscopy for acid-fast bacilli (AFB) - negative |
| - | 04/15/2019 | |
| Biochemistry | 04/16/2019 | • Transamiases -Glutamate-pyruvate transaminase:22 UI/l (vr:8-49 UI/l) - Aspartate-Amino-Transférase: 31 UI/l (vr: -48 UI/l) - Phosphatase alcalin: 68 UI/l (vr:40-129 UI/l) - Gamma-Glutamyl-Transpeptidases: 72 UI/l (vr: 7-32 UI/l) • |
| Pneumology | 04/26/2019 | • Bronchic fibroscopy - None suppurative inflamatory bronchial |
Haematologically, the patient of the AB Positive blood group. Blood count was normal, an increase in the marker of inflammation was observed. Biochemically, no failure was observed for the kidney functions.
Bronchial fibroscopy revealed non-suppurative left bronchial inflammation. The bronchial suction fluid was analyzed and was unsuccessful.
The patient's sputum was analyzed to visualize numerous Paragonimus-eggs with an average of 74/39 μm for 17 eggs studied, as shown in Fig. (1).
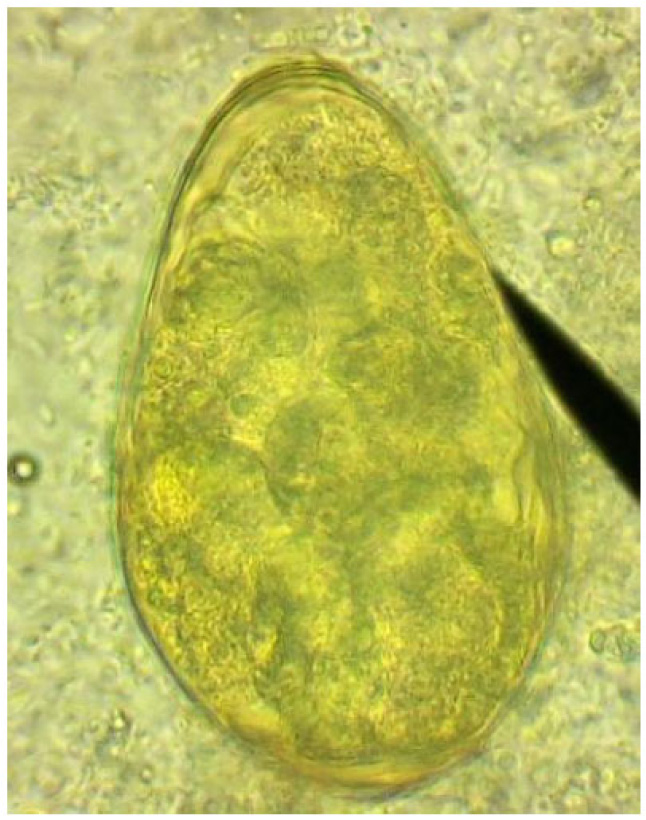
Detection of acid-fast bacilli in stained and acid-washed smears examined microscopically did not the initial bacteriologic evidence of the presence of mycobacteria.
Subsequently, a Gene Xpert/RIF analysis of the broncho-alveolar lavage fluid made it possible to make the diagnosis of pulmonary tuberculosis.
3. RESULTS AND DISCUSSION
Pulmonary distomatosis is very common in Asia, unlike Africa, due to culinary habits, which expose them. In Africa, Nigeria is the country most affected by the cause of Nigerian- Biafran War in 1970 which led to such a famine that people were forced to consume undercooked food due to lack of energy source. Cameroon comes in second place because of traditional practices, leading people to consume crab juice, which would have virtues promoting good fertility.
Once ingested, the parasite preferentially migrates to the lungs. However, it can complete its course in any organ and give symptoms and clinical signs related to its location. when it is in the brain, it can lead to epileptic seizures.
Clinically, the majority of symptoms are cough, hemoptysis, dyspnea, chest pain.
Fever is not common symptom, and it can occur as part of a secondary infection. Digestive disorders can be observed and linked to the passage of the worm in the digestive tract.
Biologically, eosinophilia can alert the clinician. There is no particular trouble. The search for Paragonimus eggs can be carried out both in the sputum and in the stool since the patient's ingestion of eggs after the cough is possible. In children, it is important to make a gastric tube and look for eggs in the aspirated fluid.
The elevated liver enzymes observed in this study should be related to the alcoholic status of the patient. In paragonimiasis disease, this picture is not frequent.
Serological tests exist but do not make it possible to specify whether it is a new or old infection. Antibodies take a long time to disappear from the circulating blood.
Regarding parasite species, variabilities certainly exist in Africa and are shown in the study by Cabaret et al., which indicates four species in fact [14]. Progress has been made in species’ identification in Cameroon and Nigeria. However, in Côte d'Ivoire, the species’ questions are not yet elucidated. Complementary work will settle the question definitively.
Therapeutically, the preferential drug for the treatment of Paragonimiasis is Praziquantel with a 2-days regimen a daily dose of 3 × 25 mg/kg orally., the mechanisms of action are not yet fully understood [15]. The side effects of this molecule are well documented and usually tolerated by patients, in particular the metallic taste. However, other alternatives exist, in particular with Triclabendazole, Bitionol. In the case of paragonimiasis at the complication stage, surgery is sometimes required to root out the adult forms.
CONCLUSION
Paragonimiasis is endemic in Côte d’Ivoire. The management of patients suffering from chronic cough characterized by hemoptysis or not should not obscure the differential
diagnosis with paragonimiasis. During the patient’s interrogation, the clinician should always keep an open mind and seek crabs’ consumption, particularly in endemic areas exposure. Furthermore, the systematic Paragonimus eggs detection reflex in the sputum or stools should be planed. The strengthening of human resources, as well as the improvement of the technical platform of reference laboratories in regions in order to identify Paragonimus eggs, are urgently needed.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics committee of the Laboratory Search Committee under Code #. 007/RC-04PA 2021.
HUMAN AND ANIMAL RIGHTS
No animals were used in the study. All the reported experiments involving humans used in the study were in accordance with the ethical standards of the committee responsible for human experimentation and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from the patient involved in this study.
STANDARDS OF REPORTING
CARE guidelines and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the conclusions of this study are available from the Laboratory of Parasitology-Mycology, Faculty of Medicine, Abidjan Côte d'Ivoire. The personal and medical data of the patient are not accessible to public. However, data are available from the author [N. A.D.A.] upon reasonable request and with the authorization of the Faculty of Medicine of Abidjan, Côte d'Ivoire.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank Professor AHUI, J.M. from Teaching Hospital of Cocody, Abidjan, Côte d’ivoire.


