Pediatric Cutaneous Leishmaniasis in a Private Clinic of Abidjan, Ivory Coast: A Case Report
Abstract
Introduction:
Cutaneous Leishmaniasis (CL) is a zoonotic disease with global distribution, especially in underdeveloped countries. This parasitic disease is caused by the bite of an infected sandfly.
Case Report:
We report here the first case of cutaneous leishmaniasis discovered incidentally in an 11-year-old child in a private clinic. He is a primarian boy who had a wound located on his left leg. On questioning, we ascertained that the wound was not the first one and had been present for about 2 months. The cutaneous lesion was painless but itchy. The location, the crateriform appearance, and the chronic nature of the wound led us to suspect a case of cutaneous leishmaniasis.
Methods:
Microscopic examination of cutaneous exsudation’s smears of lesions revealed amastigote forms of leishmania, confirming our diagnostic hypothesis. The child was placed under Imidazole-based treatment associate cloxacilline. The child also received local gentamycin-based dressings.
Results:
The child was cured after one month. The diagnostic issues of cutaneous leishmaniasis constitute a great challenge for practitioners in endemic zone. Also, a systematic differential diagnostic should be required in the face of chronic wounds.
Conclusion:
The integration of the screening and management of cutaneous leishmaniasis against Buruli ulcer are eagerly waited as a future work.
1. INTRODUCTION
Cutaneous leishmaniasis is a parasitic disease caused by an infected Sandfly [1]. This disease is characterized essentially by skin damage without spreading to the deep organs or to the mucous membranes [2]. Cutaneous leishmaniasis is the most common leishmanial syndrome worldwide [3]. Cutaneous Leishmaniasis (CL) is a significant global public health issue. Leishmaniasis affects 350 million people globally in 88 countries, with approx. 12 million in a debilitating condition from symptoms [4]. The 2015 Global Burden of Disease study estimated 3.9 million prevalent CL cases from 2002 to 2015 [5]. The majority of CL is reported from Afghanistan, Algeria, Brazil, Colombia, Pakistan, and the Syrian Arab Republic [6]. This tropical disease is due to genus Leishmania. Leishmania spp. and Trypasosoma spp belong to the Trypanosomatidae family [7]. In Africa, the majority of cases are distributed in the Maghreb countries, while sporadic cases are screened in Sub-Saharan States [8]. In Côte d’Ivoire, no case of CL have been reported in private health centers. We report here a case of cutaneous leishmaniasis in a child.
2. CASE REPORT
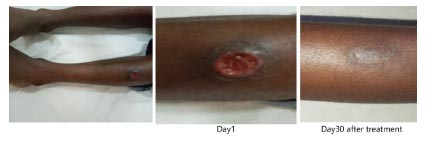
Our case concerns a child 11 years old, male, in primary school, originally from Cote d’Ivoire, resident in San Pedro (N 7°32’; E 5°32’) two years ago. The child two months ago presented with a cutaneous rash ” bouton” to the anterolateral face of the left leg. Ten days later, we noticed the bouton had ruptured, and some serosities came out of the sore. A few weeks later, the size of the wound began gradually to enlarge. This wound was not very painful. In addition, two other places of the bilateral legs were affected. The child didn’t receive adequate care at health facilities in the locality where he resided. His friends at school were very suspicious of him due to the aspect of the wound. On the psychological level, the child was greatly disturbed, so much that he experienced an episodes of sadness bordering on depression. During the school holidays, the child went to stay with his parents in Abidjan, where his aunt arranged him to attend a private clinic for his care. The patient consulted on June 26th, 2020 the Group Medical and Consulting Bozouma (GMC-BOZOUMA) Clinics for wound dressing. The appearance of the wound captured our attention, and the child was referred for consultation.
The clinical examination revealed the following information:
• General Examination: Good general condition, normal consciousness, conjunctivae slightly colored, saburral tongue.
• Temperature: 98.6 F, Pulse: 91 bpm, Weight: 38 kg, Height: 1.15 m, BMI: 28.73
• Cardiovascular System: regular tachycardia without any added murmur. Various arterial pulses were well received.
• Pleuro-pulmonary System: at auscultation, the lungs were clear, good air entry bilaterally
• Digestive System: the abdomen was soft, not tender, and without hepato-splenomegaly.
• Other Systems: No lymphadenopathy of the lymph nodes. However, we noticed the presence of 2 small wounds, about 0.8 and 1.2 cm of diameters on the right leg.
The main wound was ulcerative, 4.8 cm in diameter, located on the upper anterolateral side of the left leg, as shown in Fig. (1) There was induration of the edges of the ulcer and a crater consisting of hematic areas.
In the presence of this image strongly reminiscent of cutaneous leishmaniasis, a proposal was communicated to the aunt and the child's consent was recorded.
3. METHODOLOGY
As a methodology, three blood smears and two smears of exudation of the lesions were performed. Subsequently, the slides were stained with the May Grünwald Giemsa method [9]. In addition, a biological control was carried out on two blood tubes, including one EDTA tube collected to check the hematology, kidney, and hepatic functions. Some additional slides were prepared and sent the same day to the Parasitology-Mycology Laboratory of the Abidjan School of Medicine for confirmation. In addition, other samples were prepared and conditioned with the agreement of the aunt and sent to the Pasteur Institute of Algiers (Algeria) for species identification of parasites.
4. RESULTS
The result was fructuous. Indeed, it has shown us the possibility to highlight “amastigote” ovoid forms of Leishmania. The size of leishmanias was ranged to 3-4 microns diameter as shown in Fig. (2). Otherside, the child’s biological tests were all normal,

Concerning the treatments, he received antibiotic coverage (Oxacillin 500 mg: 1 tab x3 / day for 2 weeks) to prevent bacterial infections and wound complications; and an Imidazole drug (Metronidazole 500 mg: 1 tab x3 / day for 4 weeks. The wounds were dressed with a gentamycin solution as recommended by some authors and empirically used in our teaching hospital [10]. The treatment was successful, with the reduction of the size of the wounds followed by scarring one month later. A smear checking was negative two months later. The good adherence to the treatment was due to the absence of adverse events.
5. DISCUSSION
The lesions which result from CL are observed mainly on the exposed parts of the body [11]. The majority of CL cases are seen in childhood, and in the epidemiological view, it has been shown that children are more at risk than adults [12-14].
The clinical manifestations of CL depend on both host immunological responses and parasite virulence [15]. In addition, co-infection of CL strains acquired from different species of Leishmania is possible [16]. It is important to keep in mind that mucocutaneous or mucosal leishmaniasis and visceral leishmaniasis could occur after several months or years after a CL infection [17].
Environmental factors and socio-economic conditions are important factors which is to take into account the presence, distribution, and diversity of sandflies [18]. Many studies have explored the impacts of rainfall, temperature, and humidity on zoonotic CL infection [19 , 20].
In terms of vectors, potential Phlebotomus were recorded in 2019 as vectors of CL in Cote d’Ivoire. Indeed, a study in three villages of the city of Bouaké concluding that the genus Sergentomyia constituted 91% of the sandfly taxa present, versus 9% for the genus Phlebotomus. The species of Phlebotomus present included Ph. bergeroti, Ph. rodhaini and Ph. sergenti; and thirteen species were found in the genus Sergentomyia [20].
Concerning epidemiology, the first case of CL in Cote d’Ivoire was detected seven years after independence in 1967 [21]. Three cases were subsequently diagnosed 15 years later. One patient was from Burkina Faso who presented with an ulcerative lesion about 2 cm in diameter located in the lower third of the left leg. The second patient concerned a native of the Western region of Cote d’Ivoire and the third was from the Eastern region. They presented ulceration of 3-5 cm in diameter at the top of the skull and a dry psoriasiform lesion of approximately 1.5 cm respectively [22]. A high number of cases of CL was in 1986 detected in six patients [23]. Twenty-two years later, nine among 20 persons from the villages of Boblénou, Kouadio-Miankro and Golikro who suffered from cutaneous lesions were found positive after biomolecular tests in the district of Bouaké northeast [24].
In terms of which Leishmania species cause cutaneous leishmaniasis in Cote d’Ivoire, few information is available. All the studies available have not determined which species are responsible for C.L. in Ivory Coast. The only study identifying the species is the first record of L. infantum in dogs, from 2020 [25].
As far as treatment is concerned, it will depend on several factors (including the aspect and the size of the lesion, the localization, and the number of the lesions) [26]. Local skin therapy could be based on a curettage removal of all inflamed tissues. Otherwise, cryotherapy using liquid nitrogen could be used, or local heat therapy. Treatment with topical creams or intra-lesional injection of pentavalent antimony drug is possible for uncomplicated CL. Nowadays, no vaccine exists to prevent CL in the world [27 , 28]. In addition, treatments based on imidazole have been proposed as an alternative by various authors [29-31]. The Pentavalent antimonial compounds are the first- line drugs of treatment for all forms of leishmaniasis [32]. The treatment with Metronidazole combined with Gentamycin administered locally to our patient allowed to cure the disease of that child without major adverse effects.
The strength of our study was due to the isolation of the parasite on direct examination. However, one of the limitations could be the impossibility of biomolecular identification of the species.
CONCLUSION
Cutaneous leishmaniasis occurs most of the time in children. Any chronic sore should be suspected of CL, if a context of no traumatism cutaneous lesions is described in endemic zone. The impact of psychological issues resulting from leishmaniasis infection is not negligible. Due to the presence of Burili’s ulcer foci and considering the similarity of skin lesions between CL and Burili ulcer, the integration of screening and management of this disease into the Buruli ulcer program would be essential. Also, the strengthening of human resources and the improvement of technical platforms of laboratories at a regional level in this country is strategically recommended in order to diagnose cutaneous leishmaniasis and visceral leishmaniasis.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethical Comitee of the Group Medical and Consulting Bozouma (GMC-BOZOUMA) clinic, Abidjan, Côte d’Ivoire, under approval No. 2020-0003/ DA/CI.
HUMAN AND ANIMAL RIGHTS
No animals were used in the study. All the reported experiments involving humans used in the study were in accordance with the ethical standards of the committee responsible for human experimentation and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ ecodes/node/3931).
CONSENT OF PUBLICATION
Informed consent was obtained from the child and his aunt in charge to be involved in this study.
STANDARDS OF REPORTING
CARE guidelines and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the Group Medical and Consulting Bozouma (GMC-BOZOUMA) clinic. Still, restrictions apply to the availability of these data, which were used for the current study, and so are not publicly available. Data are however available from the author [N. A.D.A.] upon reasonable request and with permission of the Faculty of Medicine of Abidjan, Côte d’Ivoire.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank Doctor Jean Ouhon and all the staff of GMC-BOZOUMA for substantial contribution and Daniel Romero-Alvarez and Muriel Rabone for their critical comments.


