Ultrastructure-based Insights on Anti-Trichomonas vaginalis Effects of Selected Egyptian Red Sea Marine Resources
Abstract
Background:
Metronidazole is used for the treatment of trichomoniasis. However, a growing number of Trichomonas vaginalis (T. vaginalis) isolates are now resistant, which is an urgent issue to search for new alternatives. Worldwide marine pharmacy confirms the enormous potential of sea species as a source of novel pharmaceuticals.
Objective:
This study aimed to investigate the anti-T. vaginalis activities of ethanolic extracts of Red Sea marine resources, soft corals; Sarcophyton glaucum and Litophyton arboreum and methanolic extracts of Red Sea brown algae; Liagora farinosa, Colpomenia sinuosa, Hydroclathrus clathratus, and Sargassum graminifolium, as well as sea cucumber (Holothuria fuscocinerea) and sea urchin (Echinometra mathaei). T. vaginalis growth inhibition was determined using 2 concentrations for each marine extract 10 and 100 µg/ml in comparison to media control. Drugs that showed good initial activity were further tested to calculate their IC50 in comparison to metronidazole. The ultrastructural impact of the more effective extracts was further assessed.
Results:
H. clathratus, L. farinose, sea urchin E. mathaei and sea cucumber H. fuscocinerea reduced the growth of T. vaginalis effectively and showed high activity with IC50 of 0.985±0.08, 0.949±0.04, 0.845±0.09 and 0.798±µg/ml±SD, respectively. Concerning microscopic analysis, marine extract and metronidazole-treated cells presented similar morphological changes. The nuclear membrane was damaged, the nuclei were dissolved, the rough endoplasmic reticulum was widened, and the chromatin was accumulated. In the cytoplasm, numerous autophagic vacuoles appeared, the organelles were disintegrated, the flagella were internalized and hydrogenosomes with altered morphologies were observed. The cell membrane was partially damaged, with cytoplasmic leakage and cell disintegration.
Conclusion:
This study describes the report on the activity and morphological changes induced by Egyptian Red Sea marine resources against T. vaginalis. The results obtained herein presented new opportunitiess. Further, bio-guided fractionation and isolation of active compounds are needed.
1. INTRODUCTION
Trichomoniasis, caused by the flagellate protozoan Trichomonas vaginalis (T. vaginalis), is the most common sexually transmitted disease in the world with the highest rate of incidence, 276 million new cases yearly [1]. Although curable, it may cause severe health consequences especially in females such as infertility, human immunodeficiency virus acquisition, cervical cancer and adverse pregnancy outcomes leading to premature rupture of placental membranes, premature labor and low-birth-weight [2]. Though generally asymptomatic in men, yet its association to cancer prostate is alarming [3].
Metronidazole, the drug of choice has shown clinical resistance as early as in 1962 [4]. It is estimated that approximately 2.5-10% of all cases display resistance. Because of the lack of approved alternative treatments, those resistant cases are therefore usually treated with increased doses, which may lead to side effects [5, 6]. These side effects may result in treatment discontinuation, leading to further spread of infection and emergence of more resistant strains [7]; therefore novel therapeutic alternatives for the treatment of trichomoniasis are crucial [8].
Marine resources, through evolution, had to develop characteristics to cope with the changes in the environment, namely extreme conditions of temperature, pressure, and exposure to high concentrations of pathogenic bacteria, fungi, and viruses. Many of these adaptations resulted in the production of compounds that revealed interesting activities in the pharmacological field of cancer, acquired immune deficiency syndrome and arthritis [9].
Seventy-five marine compounds were reported to have antidiabetic, anti-inflammatory activities and affect the immune and nervous system. In fact, several marine compounds were approved by the Food and Drug Administration and are currently available to the public as drugs for leukemia, metastatic breast cancer, and Hodgkin's disease [10].
Several research groups around the world have paid attention to discover natural products from marine organisms with activity against protozoal diseases, such as malaria, Chagas disease, leishmaniasis, and African trypanosomosis [11-13]. Anti-Trichomonas activity was also described in studies on marine algae [14-16] and fungi [17]. Extracts from twenty-five seaweeds were tested against T. vaginalis, of which 44% presented high to moderate activity with low cytotoxicity [14].
The Red Sea is a hot spot for marine biodiversity. Approximately 40% of the marine species identified worldwide are native to the Red Sea [18].
In recent years, a number of studies have proved that the bioactive compounds isolated from Red Sea marine organisms exhibited pesticidal [19], antioxidant [20], antimicrobial and anti-inflammatory properties [21, 22]. The research on natural products, isolated from Red Sea marine extracts, with antiparasitic activity is very limited [23, 24] although these products may be a suitable alternative to synthetic drugs in the future as they are relatively safe, biodegradable, and are easily available [25].
Given the need for anti-Trichomonas novel therapies and the emerging importance of marine-associated extracts as a source of bioactive molecules, this study aimed to shed light on the activity of eight Egyptian Red Sea marine resources; ethanolic extracts of two soft corals; Sarcophyton glaucum (Quoy and Gaimard, 1833) and Litophyton arboreum (Forskal, 1775) and methanolic extracts of four red and brown algae; Liagora farinose (Lamouroux, 1816), Colpomenia sinuosa (Derbès and Solier, 1851), Hydroclathrus clathratus (Howe, 1920) and Sargassum graminifolium (Agardh, 1820) as well as sea cucumber; Holothuria fuscocinerea (Jaeger, 1833) and sea urchin; Echinometra mathaei (Blainville, 1825) on T. vaginalis growth. Ultrastructural analysis was employed to clarify their effect.
2. MATERIALS AND METHODS
This study was conducted at the Parasitology Research and Diagnostic Laboratory unit, Parasitology Department, Faculty of Medicine, Ain Shams University, Egypt, from May 2016 to March 2018.
2.1. The Marine Extracts
The marine resources were kindly provided by Professor Dr. Hans-Georg Breitinger, Head of Biochemistry Department at the German University in Cairo,Egypt. They were collected and identified from the Red Sea intertidal zone of Hurghada coast.
50 g of each marine soft coral extracts were macerated in 100 ml of 70% aqueous ethanol and then maneuvered [26]. The red and brown algae were rinsed with sterile water to remove any associated debris then kept in shade for a week. Lyophilized samples (15 g) were macerated in 100 ml of dichloromethane: methanol (7:3) for a whole day at room temperature and then the extract was prepared [14]. The sea cucumber (H. fuscocinerea), was freshly harvested and rinsed with distilled water to remove debris. Its body wall was cut into pieces and dried at room temperature in dim light before being ground to a fine powder. Pieces of the body wall were macerated in 300 ml of methanol-water (50:50) and the extract was prepared [27]. The sea urchin extract was prepared from live specimens (E. mathaei) and their dissected ovaries were preserved in 100% methanol [28]. All the prepared extracts were stored in dark bottles at 4°C until used.
2.2. The Culture of T. Vaginalis
Motile trophozoites were isolated, pooled and examined microscopically from vaginal washouts of patients attending the outpatient clinic, Gynecology and Obstetrics Hospital, Ain Shams University [29]. Trichomonads were in vitro cultured. Organisms exhibiting motility and normal morphology during the logarithmic growth phase were collected, centrifuged, washed three times with phosphate-buffered saline (PBS) 1x (pH 7), and resuspended in a new medium then sub-cultured every 48 h [30].
2.3. In vitro Susceptibility Assays
For the initial screen of the activity of the marine extracts, they were dissolved in 1 ml of dimethylsulfoxide (DMSO) and added to microtubes containing 1.5 ml of medium to reach concentrations of 10 and 100 µg/ml for each extract. The solutions were inoculated with T. vaginalis to obtain 4×104 trophozoites/ml and then were incubated for 2 days at 37◦C. In the tubes of control cultures, the extracts were replaced by water. After incubation, viable cells were identified and counted microscopically using a hemocytometer based on the morphology and motility and trypan blue dye exclusion where samples were mixed with trypan blue (0.2%) in equal volumes. Results were expressed as the percentage of living organisms compared to controls and marine extracts that resulted in the same growth inhibition percentage at the two concentrations were excluded from the secondary screening [31].
In the secondary screening, the selected marine extracts at concentrations of 3.1, 10, 31.6, 100, and 316.2 µg/ml were tested in duplicates to determine their 50% inhibitory concentration (IC50) in which 50% of T. vaginalis cells were alive and 50% were dead [32]. Culture and readout were carried out as described in the first round of screening. Each test included metronidazole (1 µg/ml) as a positive control, a solvent control (culture medium plus trophozoites and DMSO), and negative control (culture medium plus trophozoites). The IC50 was calculated for each treatment using probit analysis (Graph Pad Prism 4 software). The trophozoites in each tube were sub-cultured in a new medium for another 2 days, without antiprotozoal drugs or extracts. Trophozoites treated with the selected extracts from the second screening were subjected to the ultrastructural study.

2.4. Transmission Electron Microscopy (TEM)
Untreated and treated trophozoites with the selected marine extracts as well as metronidazole were sequentially fixed for 1h at room temperature in a (v/v) solution containing 2.5% glutaraldehyde and cacodylate buffer (0.1), pH 7.2. Fixed samples were washed twice in PBS and fixed with 1% (v/v) osmium tetroxide in 0.1 M cacodylate buffer for half an hour, pH 7.2, at room temperature and dehydrated in increasing concentrations of ethanol, followed by final dehydration in 100% propylene oxide. Samples were embedded in Epon 812. Thin sections were cut and processed [31]. An average of 40-50 fields of each preparation was examined.
3. RESULTS
The primary screening showed an inhibited growth of T. vaginalis in vitro in different extents. Of the eight tested marine extracts, S. glaucum, L. arboreum, C. sinuosa, and S. graminifolium showed the same growth inhibition percentage at the two concentrations 10 and 100 µg/ml and were excluded from the second screening (Fig. 1).
The IC50 from H. clathratus, L. farinose, E. mathaei and H. fuscocinerea were; 0.985±0.08, 0.949±0.04, 0.845±0.09 and 0.798±0.10 µg/ml±SD, respectively. Due to the lack of data on anti-trichomal activity scores from marine resources extracts, we define high activity as IC50 ≤ 5 µg /ml, moderate activity as IC50 5.1–10 µg/ml and low activity as IC50 > 10 µg/ml [14]. The results revealed high anti-trichomonal activity from the tested marine extracts. None of the extracts assayed were more active than metronidazole (IC50; 0.04 µg/ml± SD).
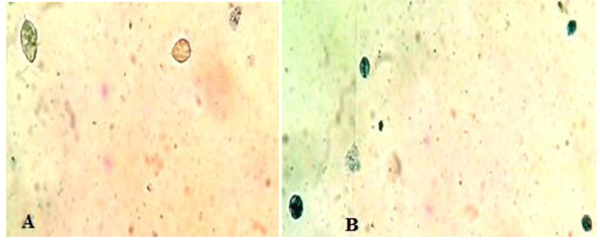
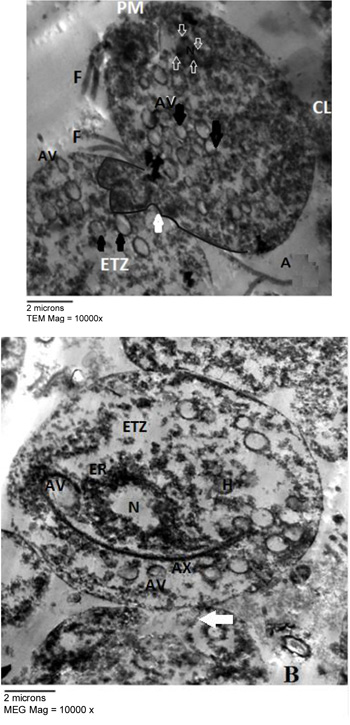
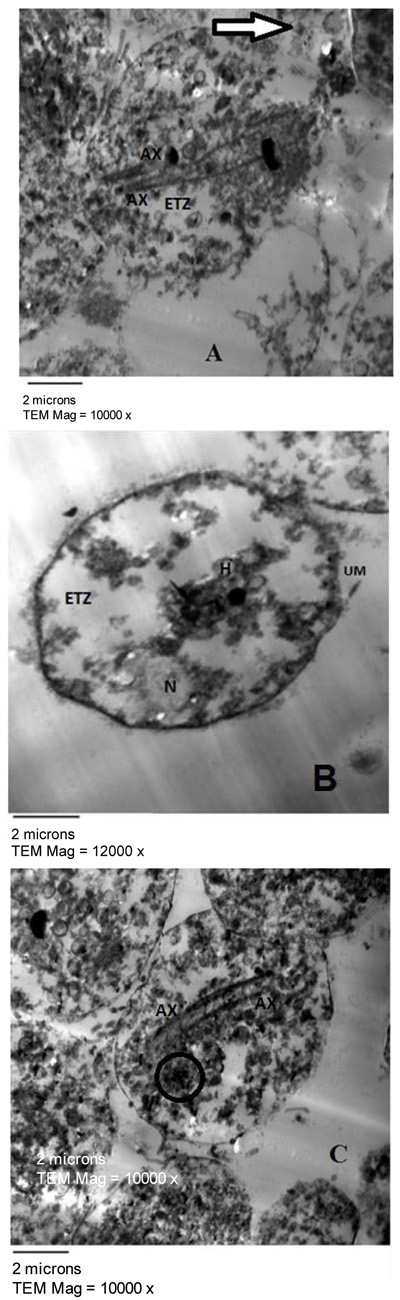
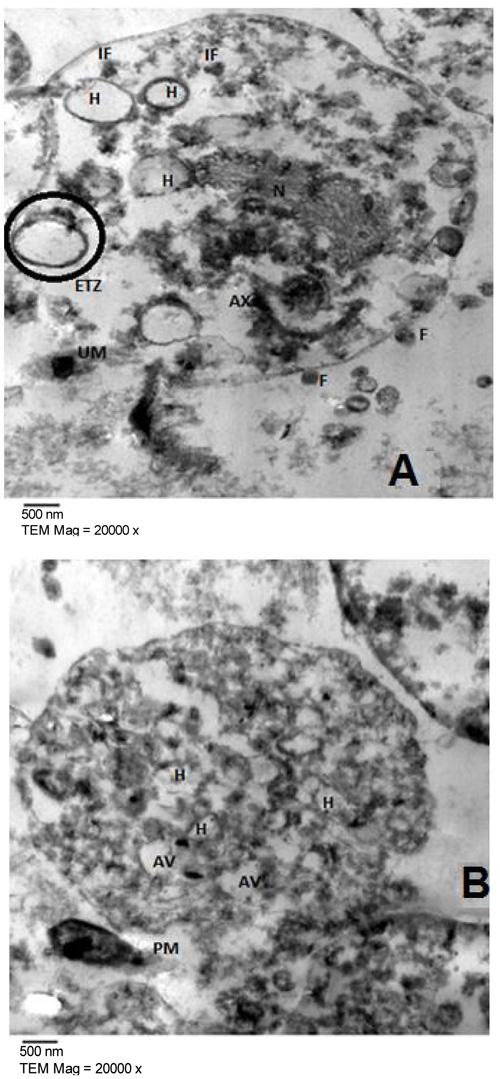
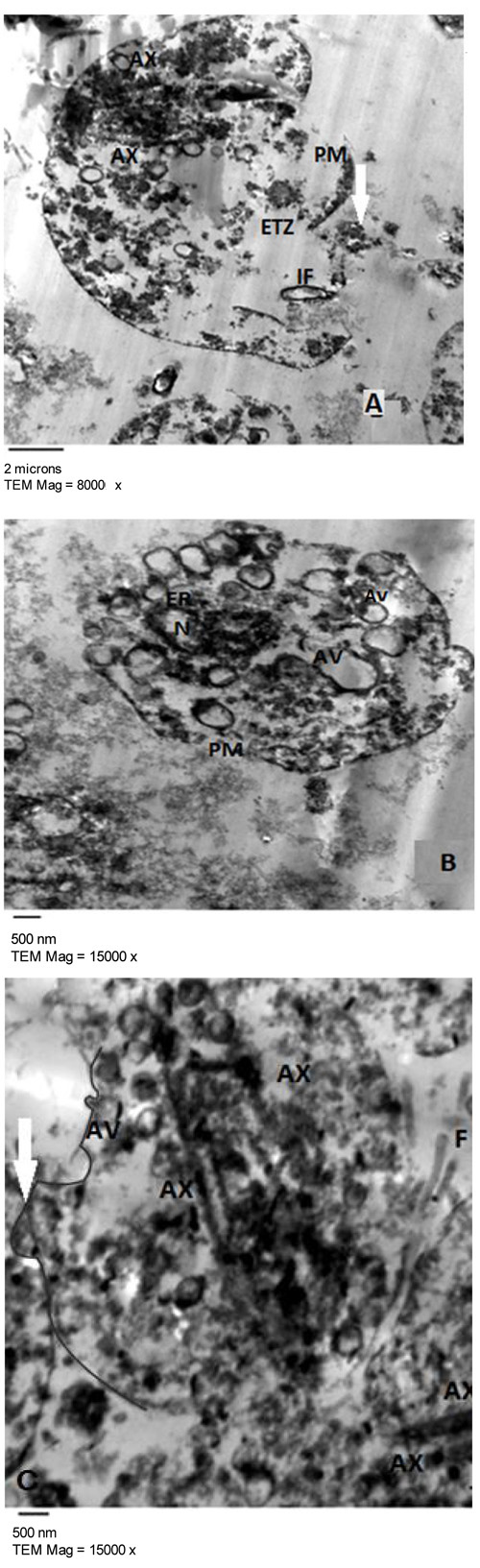
Fig. (2) represented the light microscopy of T. vaginalis trophozoites treated with 100 µg/ml of E. mathaei. Parasites were stained with trypan blue dye. Viable parasites appeared unstained while non-viable parasites appeared stained. The TEM fine structure of T. vaginalis can be seen in Fig. (3) representing the trophozoites without any treatment (negative control), Figs. (4-7) showed the trophozoites incubated in L. farinosa, H. clathratus, H. fuscocinerea, and E. mathaei, respectively and Fig. (8) represented the trophozoites incubated in metronidazole (positive control).


4. DISCUSSION
The initial screening of the anti-T. vaginalis activity of the 8 marine resources revealed that only four were effective in inhibiting parasite growth. None of the four extracts tested were as active as the positive control. However, it is necessary to consider that metronidazole is a pure compound and a reference drug.
From the tested red and brown algae, two species; H. clathratus and L. farinose, showed high anti-trichomonal activity with IC50; 0.985±0.08 and 0.949±0.04 µg/ml±SD, respectively. These results are consistent with previous studies that stated that brown alga Lobophora variegata presented antiprotozoal activities on T. vaginalis, Entamoeba histolytica, Giardia intestinalis, Leishmania mexicana and Trypanosoma cruzi. They approved that the whole extract of L. variegata showed high activity against T. vaginalis with IC50, 3.2 µg/ml±SD and unexpectedly when the whole extract was fractionated, fractions explicated a lower activity [15]. Also, the anti-trichomonal properties of organic algal extracts of 25 tropical seaweeds approved a high to moderate activity from 44% of the studied seaweeds. Moreover, L. variegata and Udotea conglutinata showed a maximal antiprotozoal activity with IC50 of 1.39 and 1.66 µg/ml±SD, respectively [14].
Currently, the methanolic extract of the body wall of Red Sea cucumber H. fuscocinerea showed high anti-trichomonal activity with IC50 of 0.798±0.10 µg/ml±SD. Previously, the best antibacterial and antifungal effect of the sea cucumber Stichopus variegatus was shown by the methanolic extract of the body wall in comparison to chloroform and n-hexane extracts [33]. In the same context, the anti-plasmodial capacity of sulfated polysaccharides isolated from the body wall of the sea cucumbers Holothuria grisea and Isostichopus badionotus was recorded [34]. The methanolic extract of the body wall and coelomic fluid of Holothuria leucospilota was shown to exert strong anti-leishmanial activity in experiments with Leishmania infantum tested using both promastigote and axenic amastigote forms [35].
In the present study, the methanolic extract of the sea urchin, E. mathaei showed an IC50 0.845±0.09 µg/ml±SD. Its antibacterial activity has previously been described within a wide range of echinoderm species [36]. The ovary extract of the urchin, Diadema setosum had excellent antimicrobial properties against a vast variety of pathogenic and non-pathogenic bacteria [37]. It has been concluded that the sea urchin, E. mathaei, can be a source of novel antibiotics [38].
The ultrastructural alterations viewed here demonstrated the same extent and severity of damage as compared to metronidazole, indicating that they might have the same mechanism of action.
T. vaginalis parasites treated with methanolic extracts from H. clathratus, L. farinose, E. mathaei and H. fuscocinerea showed a centripetal relocation of the flagella to the inner (more anaerobic) portions of the cytoplasm. This event may represent an early stress-induced event, prior to cell death of some parasites [39]. It was claimed that T. vaginalis, when submitted to stress conditions, internalize their flagella to form pseudocysts as an escape strategy from the environmental changes. Also, it was reported that exposure of T. vaginalis to chemicals can lead to pseudocysts formation as an irreversible process that ends up with the cell death, depending on the exposure time and/or the concentration of the chemical employed [40]. The flagella of Tritrichomonas (Tr.) foetus were internalized in an intact and functional form when parasites were submitted to stress conditions, such as variations in the environmental temperature [41]. A similar effect was observed in Giardia lamblia [42] and Tr. foetus [43] trophozoites when incubated with synthetic metronidazole analogs and thiabendazole, respectively. In these studies, trophozoites demonstrated evident ultrastructural disorganization characterized by the internalization of the cytoskeletal components of the flagella and adhesive disc in the cytoplasmic matrix.
In the current study, the marine, as well as the metronidazole-treated parasites, presented an intense cytoplasmic vacuolization with reduced cytoplasmic electron density. Such vacuoles, which varied in size and morphology, exhibited a content resembling membranaceous material. Cytoplasmic vacuolization has been often associated with an autophagic process and cell death [39]. The morphological features of autophagy include cytoplasmic vacuolization, degradation of cytoplasmic ingredients with some chromatin condensation [44]. The autophagic pathway begins with the sequestration of cytoplasm in double-membrane vesicles known as autophagosomes which fuse with lysosomes, and the contents are degraded. One important outcome after treatment against Tr. foetus with griseofulvin was the presence of vacuolar contents from the flagellae and axostyle-pelta complex, suggesting an autophagic process of odd cellular contents [39]. On the other hand, there is a profuse increasing list of marine drugs reported to inhibit cancer progression through reactive oxygen species (ROS) mediated cell death and autophagy [45]. ROS lead to irreversible oxidative damage to proteins, lipids, and DNA of T. vaginalis trophozoites triggering autophagy. Autophagy comprises processes for cellular homeostasis and cell death [46] and plays an important role in the recycling of ROS and can be considered an effective escape/repair system.
Marine extracts showed a substantial dilatation of endoplasmic reticulum (ER), indicating that the oxidative stress caused by treatment may have interfered in the calcium homeostasis and/or cytochrome activity of the trophozoites. As observed from studies on Leishmania sp., it was stated that some drugs affect the cytoskeleton and can modify the ER [47].
A frequently observed event in both marine extract and metronidazole-treated parasites was the formation of numerous surface interdigitations among adjacent trophozoites, indicating extensive cannibalism associated with necrotic areas and plasma membrane discontinuity. These were frequently observed in T. vaginalis, surviving cells can feed on the nutrients released by dead sibling cells or on attacking the cells per se [48]. Microbial cannibalism was reported in ciliated protozoa and flagellates [49, 50].
TEM showed that some of the marine and metronidazole- treated trophozoites drastically changed their morphologies, presenting some multiplicated organelles (axostyles). Several authors have observed such abnormal forms when T. vaginalis and Tr. foetus were under drug pressure [39, 51, 52]. Moreover, it was reported that vinca alkaloids produced a reversible block of cytokinesis in Trypanosoma cruzi with the presence of multiple nuclei and kinetoplasts [53].
Accumulation of numerous masses of glycogen was observed in marine extract treated parasites. Parrou et al. (1997) mentioned that glycogen production and accumulation in yeasts can reach up to three times the basal level in response to thermal, hyperosmotic shock or oxidative stress which may be an adaptive mechanism for acquisition of the reserve carbohydrates such as glycogen [54, 55].
Concerning hydrogenosomes, treatment with marine extracts induced a random distribution with the appearance of an irregular matrix, as well as eliciting of a significant increase in volume and decrease in number [56]. The metronidazole is activated to its cytotoxic form in the hydrogenosomes of T. vaginalis and Tr. foetus and the resistant cells had approximately one-third the size of hydrogenosomes in comparison to the drug-sensitive ones. When hydrogenosomes suffered irreversible damage they underwent an autophagic process and were destroyed [57]. Hydrogenosomes are the key organelles involved in the carbohydrate metabolism of T. vaginalis [58]. Because they are of low redox potentials and the marine extracts are ROS generating drugs [45], it can be considered that the hydrogenosomes observed here might have been partially destroyed due to a direct impact of the marine extracts. They are considered an excellent drug target because their metabolic pathway is distinct from those found in mitochondria, and thus, will probably spare the host cells [57].
Eventually, we presume that the damaged cytoplasmic membrane and organelles might cause Trichomonas trophozoites to die. Similar morphological aspects of T. vaginalis treated with pro-apoptotic drugs such as nuclear fragmentation, rounded shape, cytoplasm vacuolation, and apoptotic-like bodies were documented [59]. Moreover, after treatment with different H2O2 concentrations, rounded shaped Tr. foetus was found with, peripheral masses of chromatin, and elongated hydrogenosomes with an electron-dense matrix, large-sized vacuoles with altered contents, internalized flagellae, ER proliferation, and deformed axostyle [40].
To further understand the exact mechanism of cell death in T. vaginalis, additional molecular studies are necessary. Marine products may contain a defense toxin for its marine organism’s body, utilizing these compounds for pharmacological use is conditioned by its toxicity profile that would hinder introducing it for clinical trials if not in safe levels. Further chemical analysis on the present extracts with high anti-trichomonal activity would definitely reveal the important chemical and structural components responsible for these biological activities, in view that the potent antimicrobial effect of marine organisms resides in the efficiency of the extraction method [60], and the solvents used [61].
CONCLUSION
These data suggest that marine extracts from the Red Sea Coast may present potential candidates for further investigation and possible use as anti-trichomonal with regard to their achievability and low cost.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Informed consent was taken from all patients before taking vaginal samples.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared None.


