All published articles of this journal are available on ScienceDirect.
Effect of Etidronate and Ibandronate on Cytosolic Ca2+ in HT29 and Parasite Cell Line from Echinococcus Granulosus sensu lato
Abstract
Background:
The bisphosphonates are synthetic analogs of pyrophosphate in which two phosphates are connected through carbon instead of oxygen. They are approved compounds for the treatment of hypercalcemia, bone diseases and they have been proposed to treat infectious diseases. Bisphosphonates’ main mechanisms of action are on calcium metabolism, inhibition of protein prenylation and on ATP synthesis. In a previous work, the antiparasitic activity of bisphosphonates on a cell line from Echinococcus granulosus, sensu lato protoscoleces, 30 µM etidronate and ibandronate have antiproliferative activity after 72 h of incubation, decreasing intracellular ATP and only etidronate increased intracellular total calcium concentration.
Objective:
This work studied the effect of etidronate and ibandronate on cytoplasmic ionic calcium concentration in parasitic cell line and in HT29, cell line from human colon adenocarcinoma.
Methods:
Ionic calcium was measured by spectrofluorometric, labeling cells with Fluo-4AM. Cells were suspended in Na+ or K+ rich buffer and two calcium salts were used Cl- or Gluc-, anion permeable and impermeable, respectively.
Results:
Remarkable differences between cell lines were shown with the effect of bisphosphonates on intracellular ionic calcium concentration in hyperpolarized cells and these differences were smoothed on depolarized cells, in spite of the similar cellular response to calcium salts in absence of bisphosphonates.
Conclusion:
The bisphosphonates, mainly etidronate, decreased intracellular ionic calcium on parasitic cells explaining other aspects of their antiproliferative effect. Results suggested that other mechanism, such as Cl- and Na+ interchange are differentially affected by bisphosphonates, depending on cell line origin.
1. INTRODUCTION
The bisphosphonates (BF) are synthetic analogs of pyrophosphate in which two phosphates are connected through carbon instead of oxygen. The BF binds to bone by the chemical group P-C-P while the NH3- and lateral chains confer them with other pharmacological properties. They are approved compounds for the treatment of hypercalcemia, bone diseases and osseous cancer metastasis acting on pH regulation, hydroxyapatite stability [1] and Ca2+ metabolism [2, 3].
The BF have been proposed to treat infectious diseases [4, 5]. In our laboratory, we are studying a parasitic worldwide distributed diseases caused by Echinococcus granulosus, sensu lato (E.granulosus,s.l), an etiological agent of Cystic Echinococcosis (CE). The E. granulosus, s.l development requires two hosts: the embryonic eggs originated by the worm stage in the dog intestine released with the feces, preserved in humid environments to find the intermediary ungulate host (ovine, bovine, camelid and porcine) or humans, who ingested the eggs. The embryos invade the hosts’ organs by passing through the intestinal barrier; there they develop the larvae disease, CE. The metacestode, larvae, is located mainly in the liver and the lungs and could primarily be implanted or disseminated to other organs by spreading embryonic corps, protoscoleces (pe). Metacestode develops a cyst, external laminal membrane is rich in calcium and, inside the hydatid fluid (HF) are high-calcified isolate cells. Membrane calcification is associated with parasite viability lost [6] affecting membrane permeability [7].
The pharmacological effect of five BF was studied in our laboratory on an in-vitro parasite model constituted by a cell line, EGPE, isolated from bovine’s pe, genotype G1 by Cytochrome c oxidase, subunit 1 (DCO1) sequence [8]. EGPE forms cystic colonies in agarose and BF decreased colonies development and ATP synthesis. Nevertheless, BF effect has not always correlated with the total intracellular calcium increased. Cells treated with 30 µM etidronate (HEDP) showed total intracellular calcium increased, but cells treated with ibandronate (IB) did not, they were measured with the colorimetric method [9].
In order to investigate if the antiproliferative effect is dependent on BF role on cytoplasmic ionic calcium ([Ca2+]c) we studied the effect of HEDP and the IB on [Ca2+]c. As a number of many intracellular factors influence the [Ca2+]c, this work has been performed in whole cells assay, changing cell membrane polarization with highest extracellular [Na+]o, hyperpolarized, or [K+]o, depolarized. The osmolality was modulated challenging the cells with CaCl2 and Ca Gluconate (CaGluc2). Results obtained with EGPE cells has been compared with those obtained with HT29 cells, a cell line from human colon adenocarcinoma. The BF effects on cytoplasmic ionic calcium concentration (BF-[Ca2+]c) could be related to the pharmacological effects on cell viability.
2. MATERIALS AND METHODS
2.1. Cell Culture and Cell Growth Assay
The HT29 cells donated by Dr. Eduardo Cafferata, Leloir Institute, Buenos Aires, were cultured in DMEM- F12 (Sigma Aldrich) culture medium, 1% penicillin/ streptomycin, 2 mM glutamine (Sigma Aldrich) and 3 g/ L D-glucose (Cicarelli, Argentina), supplemented with 10% fetal bovine serum (FBS) (Internegocios, Argentina). A 5E+03 cells/ well were grown on 96 wells plate and BF treatment began 24 h after seeding. A 30 µM BF, HEDP or IB (Gador SA, Argentina) or Hank's balanced salt solution (HBSS) control sample, were added to culture medium. Viability was measured by trypan blue exclusion test up to 7 days. The EGPE cells viability were studied by cell colonies formation, seeding 1E+02 cells/ well in 2% agarose [8]. BF or HBSS treatments began on the day of seeding with the addition of BF on liquid phase: 199 Medium (Sigma Aldrich), antibiotics, sodium pyruvate, β-mercaptoethanol and 10% FBS [9]. Colonies were observed after 7 days of incubation under 400x field, inverted microscope. A number of colonies were counted and colonies size, were analyzed by MOTIC images plus 2.0 ml, Chine. Colony’s surface (µ2) was calculated by [(Width1/ 2* Width2/ 2) * π.
2.2. Measure [Ca2+]c and Incubation Conditions
2.2.1. Cell Suspensions
EGPE and HT29 cell lines, were washed 3 times with DPBS, then labeled with 6 µM Fluo-4,AM (Sigma) in Hanks balanced salt solution (HBSS) during 40-50 min in the incubator (37°C, 5% CO2, 95% air). When cells were used in depolarized conditions, 10 µM BAPTA was added after Fluo-4,AM. Then, cells were chilled to room temperature (25°C) in the dark, washed several times, and diluted with the measuring buffer. Cell suspensions were for HT29 1E+05 and for EGPE 4E+07 cells/ well, seeded in black 96 wells plate (Corning, Biodynamics); volume 90 µl/ well.
2.2.2. Medium Baths and Conditions
For assays performed in hyperpolarization the buffer was (mM): 10 PIPES (Sigma); 124 NaCl (Anedra); 1 KCl (Anedra); 1.3 MgCl2 (Sigma, Life science); 10 glucose (Cicarelli,): 25 NaHCO3 (Anedra); 1.22 KH2PO4 (Carlo Erba); 0.1 mM EGTA: pH 7.2 and for depolarized experiments the buffer was (mM): 10 PIPES; 1 NaCl; 124 KCl; 1.3 MgCl2; 10 glucose; 25 NaHCO3; 1.22 KH2PO4.
2.2.3. Salts Solutions
Calcium salts, CaCl2 (Anedra) and CaGluc2 (Sigma), prepared in deionized water and used at final concentration of 1.5 mM Ca2+. The BF, HEDP and IB, were prepared in HBSS, pH 7.5 and were used at a final concentration of 30 µM, the concentration had an antiproliferative effect on EGPE cells [9].
2.2.4. Cytoplasmic Ca2+ Measurement
It was performed in spectrofluorometer Glomax multifunction (Promega) heated at 25 °C. Fluorescence (Fl) was measured in extinction and emission (Ex/ Em) wavelengths, 495-516 nm, every 1.5 min. The minimum fluorescence (Flmin) or baseline, were values obtained from cell suspension (90 µl) with HBSS (10 µl) in the first 5 min. Then, any calcium salt solution was added to each well at final concentration of 1.5mM (10 µl). When fluorescence values reached the plateau, after 15-20 min, 9 µM ionomycin (io) was added (1 µl) to obtain the maximum value of fluorescence (Flmax). The total time of fluorescence recorded was 30 min and the final volume/ well was 111 µl. Measures were performed by triplicate in 3 independent experiments (Figs. 1a and 1b).
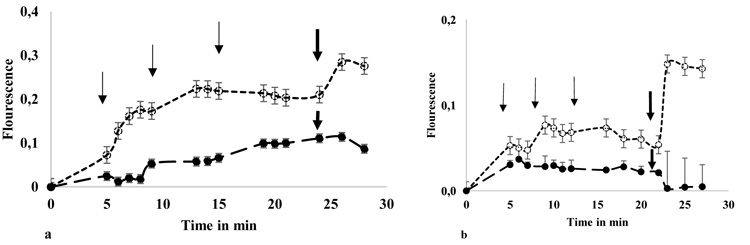
 -) instead calcium salt. Aliquots of 0.5 mM CaGluc2 was added, in samples (- -
-) instead calcium salt. Aliquots of 0.5 mM CaGluc2 was added, in samples (- - - -), as indicated in the figures, thin arrows, three times. When samples reach a plateau 9 µM ionomicin was added, in sample and control, gross arrow. Fluorescence values were obtained and expressed as Flourescence= [(Fl- Flmin)/ Flmin]. The Flmin is the last value of fluorescence obtained before calcium salt addition and Fl corresponds to the values obtained in each point of measure. Samples and controls run in duplicate and showed mean ± SD.
- -), as indicated in the figures, thin arrows, three times. When samples reach a plateau 9 µM ionomicin was added, in sample and control, gross arrow. Fluorescence values were obtained and expressed as Flourescence= [(Fl- Flmin)/ Flmin]. The Flmin is the last value of fluorescence obtained before calcium salt addition and Fl corresponds to the values obtained in each point of measure. Samples and controls run in duplicate and showed mean ± SD.
2.2.5. Studies of Bisphosphonates Effect
BF were added in sample wells and then the calcium salts were added. The values of [Ca2+]c were recorded and compared to those obtained with calcium salts without BF but, with HBSS instead, controls.
2.2.6. Net [Ca2+]c in HT29 and EGPE cells
It was calculated to compare nM [Ca2+]c between cell lines, proteins were measured by Bradford method and the values of nM [Ca2+]c / µg prot were calculated in cells defied with CaGluc2.
2.3. Calcium Concentration and Statistics
To calculate the [Ca2+]c the formula (Gee KR, 2000) was used where [Ca2+]c = Kd* (Fl- Flmin)/ (Flmax- Fl). Where Flmin was the fluorescence value obtained before calcium salts addition in the same sample (first 5 min) and Flmax was the maximum fluorescence value obtained after io addition (5 to 10 minutes) and Fl was the fluorescence value obtained in the plateau after calcium salts addition and before io (15-20 min) (Fig. 1). For the BF effect, statistic differences were calculated using contrasting samples, cells treated with BF with controls were incubated with calcium salts, cells without BF performed at the same time. The χ2 test and “student’s t” test were used to evaluate differences on BF effect on cell growth and differences on nM Ca2+/ cell or nM Ca2+/ µg proteins. Results are expressed in mean ± standard deviation (SD).
3. RESULTS
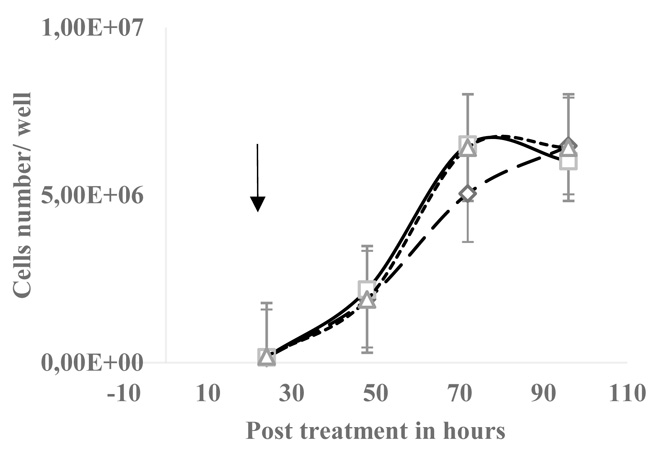
3.1. Bisphosphonates Decreased EGPE Colonies but did not Affect HT29 Cells Growth
The EGPE colonies’ size were measured after 7 days of incubation, size in µm2, mean ± SD, IB: 3.07E+04 ± 1.41E+04 and control: 9.23E+04 ± 5.36E+04 (n= 30 wells; p< 0.05, “student’s t” test). The number of colonies decreased with HEDP 14.83 ± 7.64 (n=6 wells) and IB: 18.2 ± 9.59 (n=6 wells), control colonies were 31.2 ± 9.60 (n=18); the χ2 test gave p< 0.001. On the contrary, on HT29 cell line 30 µM HEDP did not significantly affect the cells growth (Fig. 2).
3.2. The Calcium Salts Drive Cytoplasmic Ionic Calcium was Related with Polarized Cell Condition
HT29 and EGPE labeled cells were incubated in hyperpolarized cell condition and after 1.50 mM CaCl2 or CaGluc2 addition, both cell lines showed that Cl-, compared with Gluc-, significantly favored the increase of [Ca2+]c; p< 0.05, “student`s t” test, (Figs. 3a and 3b).
The difference on [Ca2+]c related to Cl- compared with Gluc-, calcium salt, in both cell lines, was lost in depolarized cell condition (Figs. 3c and 3d). However, in HT29 cells, p< 0.05 less [Ca2+]c was accounted in depolarized cells condition compared with hyperpolarized status, whatever calcium salt used, Cl- or Gluc-. Instead in EGPE cells, this difference was only found with Cl-.
3.3. Effect of Bisphosphonates on [Ca2+]c Marked more Differences between HT29 and EGPE Cell Lines.
Differences in [Ca2+]c, between both cell lines were more evident with the addition of BF, always compared samples with controls, incubated cells in the same conditions adding calcium salts. The addition of 30 µM HEDP on hyperpolarized cells condition, in EGPE, cells decreased [Ca2+]c only with CaCl2 (Fig. 3a) and, on HT29 cells [Ca2+]c increased, independent of calcium salt added (Fig. 3b).On the contrary, the results obtained in depolarized cells condition showed that HEDP decreased significantly [Ca2+]c in both cell lines, but on EGPE this effect was only observed with CaGluc2 (Figs. 3c and 3d).
The 30 µM IB smoothly differences between both cell lines, IB has never increased [Ca2+]c. In hyperpolarization, IB decreased [Ca2+]c only on HT29 cells-CaCl2 (Fig. 3b). Though, on depolarized HT29 and EGPE cells, the [Ca2+]c significantly decreased with IB (Figs. 3c and 3d). IB triggers with CaCl2, in depolarized EGPE and HT29 cells, the lowest value of [Ca2+]c (Figs. 3a-3d).
 , cells incubated with 30 µM HEDP
, cells incubated with 30 µM HEDP
 or IB
or IB
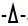 . Every point is the mean ± SD of a triplicate sample. Only the viable cells were graphed.
. Every point is the mean ± SD of a triplicate sample. Only the viable cells were graphed.
3.4. Net Difference in [Ca2+]c between Cell Lines
Intracellular ionic calcium concentration adjusted to µg of protein was for EGPE 18.36 ± 0.87 and for HT29 cells was 9.39 ± 2.25 nmol [Ca2+]c/ µg prot (mean ± SD; n= 28; p< 0.05, “student’s t” test).
4. DISCUSSION
In a previous work, the BF effect on parasite's EGPE cell line from E. granulosus G1 was studied. Bisphosphonates decreased cell proliferation and only the HEDP increased the total intracellular calcium concentration [9]. In this work, the HEDP and IB have a direct effect on [Ca2+]c in two cell lines, EGPE and mammalian HT29 cells, in the whole cell by the spectrofluorometric method. The concentration of BF used was the same of its antiproliferative effect in vitro on EGPE cells. In this work we observed a selective decreased [Ca2+]c only on parasite cell line, in hyperpolarized condition, disclosing an associated antiproliferative mechanism.
A wide range of mechanisms regulates [Ca2+]c, proteins and inorganic compounds chelate the calcium ions. These mechanisms are critical, changes in the cytosolic Ca2+concentration regulates the most of enzymes and pathways involved in normal cell physiology [10, 11]. The sources of ionic calcium are extracellular or intracellular. The intracellular calcium is released through the endoplasmic reticulum (ER), involving the inositol triphosphate (IP3) and its receptors [12]. The Ca2+ binds oxygen-bearing proteins, altering its conformation and by this mechanism distinguishes the main cation concentration, K+ or Na+, depolarized or hyperpolarized cells [13]. The Cl- facilitated the increase of [Ca2+]c on both cell lines in [Na+]o, as it has been described in endothelial cells [14] and secretory cells [15]. Ca2+-activated Cl- channels could explain the preferred calcium conductance by Cl- over Gluc-, probably by Na+/ Ca2+ pump activation in plasmatic membrane. The higher [Ca2+]c found in hyperpolarized than depolarized HT29 cells by CaGluc2, pointed out dissimilar mechanisms in [Ca2+]c regulation, involving Na+ influx, probably in response to an increase of osmolality produced by Gluc- in the extracellular space. Results obtained by Ibarra & Reisin, 1994 [16] had observed in pe more depolarization to K+ than Na+, and 50% of Na+ current was blocked by amiloride, which decreased of total intracellular calcium on EGPE cells [9]. The HF has the same concentrations of sodium, chloride, bicarbonate, and higher potassium and calcium and lower concentration of phosphate than plasma [17]. Nevertheless, potassium, magnesium, calcium, and bicarbonate were higher in pe than in HF, and chloride was not detected in pe [18], but in isolated pe membrane have described small permeability to Cl- [19]. The adaptive ionic balance of the pe to HF could explain the differences, between cell lines, of [Ca2+]c regulation related with [Na+]o and [K+]o. Other difference between both cell lines is the tolerance of EGPE cells to the ionic calcium concentration/ µg proteins, compared with HT29 cells.
For the BF, a pleiotropic action mechanism has been proposed. BF are highly ionized at physiological pH and the affinity for hydroxyapatite is favored in the HEDP over the IB [20]. On soft tissues, HEDP decreases dystrophic calcification compensating PPi deficit [21]. HEDP regulates the extracellular and intracellular [Ca2+], reduces ROS generation and cell apoptosis in glutamate injured PC12 cells [22]. For amino-BF, as IB, the pharmacological effect is related to cholesterol metabolism on protein prenylation [5, 23]. HEDP, IB and others BF need 72 h to display the antiproliferative effect [9, 24], pointing to metabolic action mechanisms, in which Ca2+ could be involved. And, in calcification of Stylophorum’s ectoderm, was described as participation of voltage-dependent calcium channel (Cav1), related with calcium L-type family [25] and BF inhibit the Ca2+ L-channel inhibitors [2].
Differences in the BF effect on cell lines were evident in hyperpolarization. On HT29 cells we observed the synergic effect of HEDP, Na+ and Cl- on [Ca2+]c and in EGPE the HEDP antagonized [Ca2+]c. But, in cells incubated with IB the cytoplasmic ionic calcium decreased to lowest values, suggesting an IB effect also on Cl- transport. Those results were in agreement with molecular selectivity of BF on HT29 cells and on EGPE, more probably due by cell molecular characteristics. The CaGluc2 with HEDP increased [Ca2+]c only on HT29 cells, suggesting an associated effect of HEDP on Na+ voltage channel, compensating osmolality produced by Gluc-. In fact, the K+ rich medium, depolarized conditions, smooth differences between cell lines on BF-[Ca2+]c, showing major molecular differences between them in plasmatic membranes. Moreover, results obtained with EGPE cells and HEDP agrees with our previous findings [9], suggesting the effect of HEDP on Ca2+ stabilization, firstly localized in plasmatic cell membrane.
CONCLUSION
The HEDP was the BF, which presented more selectivity in its effect respect to cell line, EGPE decreased [Ca2+]c incubated in hyperpolarized condition, while in the same conditions [Ca2+]c increased by the effect of HEDP on HT29 cell line. IB disclosed discrete differences between the studied cell lines, may be due mainly by its effect on intracellular membranes. BF-Cl- transport and cells response to osmolality compensated by Na+ transport require further investigation regarding obtained results using BF. The ionic calcium decrease produced by the BF in EGPE, could be one of the causes of its selective antiproliferative effect on this cell line. However, the mechanism involved in ionic calcium uptake by cells close to physiological conditions did not show strong differences between cell lines.
LIST OF ABBREVIATIONS
| BF | = Bisphosphonates |
| [Ca2+]c | = Calcium Ionic Concentration in Cytoplasm |
| CaGluc2 | = Calcium Gluconate, C12H22CaO14 |
| DCO1 | = Cytochrome c Oxidase Subunit 1 |
| HEDP | = Etidronate |
| Fl | = Fluorescence |
| IB | = Ibandronate |
| [K+]o | = More Ionic Potassium Concentration Outside the Cell in the Bath than Ionic Sodium, for Depolarization Condition |
| [Na+]o | = More Ionic Sodium Concentration Outside the Cell, in the Bath, than Ionic Potassium, for Hyperpolarization Condition |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This work was supported by a grant from Fundación Iberoamericana de Estudios Superiores (FIES), Buenos Aires, Argentina. Lilian Andrea Granada Herrera is a student of the Maestría en Investigación Clínica Farmacológica, Universidad Abierta Intreramericana, Argentina. Mariana Ferrulli is a student of Biotechnology, Universidad Nacional de Quilmes, Argentina and she obtained a fellowship from FIES to perform this work in CAECIHS, UAI. Patent pending “Linea celular de protoecolices de Echinococcus granulosus SPP. Procedimiento para la obtener estructuras quísticas y método para evaluar la actividad de drogas antiparasitarias”. Instituto Nacional de la Propiedad Industrial (INPI) Argentina P -090102320, Inventor Alicia G Fuchs, owner FIES.
Authors: Mariana Ferrulli, Fernando Gabriel Perez Rojo and Lilian Andrea Granada Herrera performed the research. Andrea Maglioco collected data and Emilio AJ Roldán contributed important reagents. Alicia G Fuchs designed the research, analyzed data and wrote the manuscript.


