Intestinal helminths in Iberian wolves (Canis lupus signatus) from Northwest Spain
Abstract
Background:
We present a study about helminth parasites in wolf (Canis lupus signatus) from Sierra de la Culebra, a protected area in the Northwest of Spain, where is the largest population of wolves of the Spanish territory and one of the largest in Western Europe.
Materials and Methods:
To this aim, 93 fecal samples were collected during May and June of 2013 using 33% zinc sulphate flotation technique and classified based on their morphology, color, structure and size.
Results:
Parasites were present in 66.67% of the samples and classified as Eucoleus aerophilus (50.54%), Strongyloides sp. (27%), Ancylostomidae gen. sp. (19.35%), Toxocara Canis (10.75%), Taeniidae gen. sp. (9.68%), Trichuris vulpis (9.68%) and Toxascaris leonina (2.15%). Their distributions were very heterogeneous with the highest prevalence being in Northwest Spain. These differences found can be attributed to local environmental factors (ambient temperature, humidity) as well as animal feeding and social behavior.
Conclusion:
A wide helminthofauna is observed in the studied wolves, similar to other studies carried out in Europe (Estonia, Finland, Italy, Latvia, Poland, Portugal, Spain and Sweden). In addition, this study constitutes the first description of the presence of Strongyloides sp. in Iberian wolf in Spain.
1. INTRODUCTION
The wolf (Canis lupus) is a predatory species which presents a wide distribution range throughout many countries in North America, Europe and Asia. This carnivore is able to survive in various habitats, thanks to its flexible feeding strategy according to the area it occupies at a given time. Furthermore, wolves are protected species in many of the regions where they live [1].
Canis lupus signatus is the subspecies living in the Iberian Peninsula. In the Northwest (Spain and Portugal), the population of wolves is large and stable, with helminth infections, being an important factor in the population dynamics of mammals [2]. Studies on the helminth fauna of the wolf in Europe are relatively scarce. In these studies, different prevalences are presented on the presence of parasites in wolves, mainly in feces (Belarus, Estonia, Finland, Italy, Latvia, Poland and Sweden) [3-9]. Furthemore, sporadically, studies have been carried out in Spain [1, 10-12] and in Portugal [10] where they have shown a significant heterogeneity of the parasitic helminth fauna in the different populations of wolves studied.
The aim of this work is the identification of the parasites base on fecal samples of Canis lupus signatus population from the Sierra de la Culebra (Northwest Spain) to try to know their status, with this being the biggest populations of wolves of Spain.
2. MATERIALS AND METHODS
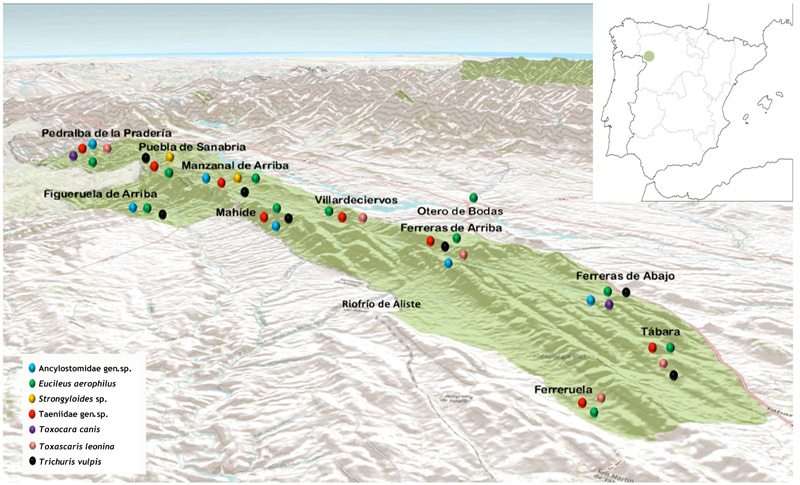
A total of 93 fresh fecal samples of Canis lupus signatus were collected and stored in individual plastic bags at 4°C from 12 locations in the Sierra de la Culebra (Northwest Spain) (Ferreras de Arriba, Ferreras de Abajo, Ferrerucía, Fugueruela de Arriba, Mahide, Manzanal de Arriba, Pedralba de la Pradería, Puebla de Sanabria, Riofrío de Aliste, Otero de Bodas, Tábara and Villardeciervos), occupied by different packs of wolves, during May and June 2013 (Table 1, Fig. 1). They were analyzed after 2-3 days of collection. Each sample was georeferenced within the municipality where it was found. The analysis was performed using 33% zinc sulfate flotation technique [11] the day after being collected. The eggs and larvae observed were identified based on their morphology, color, structure and size, following the guidelines by Foreyt [12] and Thienpont et al. [13] at 10X, 40X and 100X magnifications.
| % | Samples (n) | Ancylostomidae gen. sp. | Eucoleus aerophilus | Strongyloides sp. | Taeniidae gen. sp. | Toxocara canis | Toxascaris leonina | Trichuris vulpis |
|---|---|---|---|---|---|---|---|---|
| Ferreras de Abajo | 3 | 100 | 66,67 | 14,29 | 33,33 | - | 33,33 | 33,33 |
| Ferreras de Arriba | 7 | 14,29 | 28,57 | - | 14,29 | 14,29 | - | 14,29 |
| Ferreruela | 3 | - | 33,33 | 100 | - | 66,67 | - | |
| Figueruela de Arriba | 10 | 30 | 70 | 20 | - | - | 20 | |
| Mahide | 7 | 28,57 | 42,86 | 14,29 | - | - | - | 28,57 |
| Manzanal de Arriba | 9 | 33,33 | 55,56 | 44,44 | 11,11 | - | - | 11,11 |
| Otero de Bodas | 2 | - | 50 | - | - | - | - | - |
| Pedralba de la Pradería | 15 | 33,33 | 66,67 | - | - | - | 6,67 | |
| Puebla de Sanabria | 15 | - | 53,33 | 20 | 6,67 | - | - | 6,67 |
| Tábara | 15 | 6,67 | 60 | 33,33 | 13,33 | 13,33 | - | 20 |
| Villardeciervos | 7 | - | 28,57 | 14,29 | 14,29 | 14,29 | - | - |
To graphically represent the distribution of the analyzed samples and the positive samples, the program Arc-GIS 10.1 (ESRI, Redlands.CA, USA) was used, in which a layer was included with the cartography of the zone and the geographical references of the samples.
3. RESULTS
Of the collected samples, 61.2% contained eggs, 3.15% helminth larvae or 2.32% both. The identified parasites and their prevalence were: 19.35% Ancylostomidae gen. sp.; 50.54% Eucoleus aerophilus; 27% Strongyloides sp., 9.68% Taeniidae gen. sp., 10.75% Toxocara canis, 2.15% Toxascaris leonine and 9.68% Trichuris vulpis. There was a great geographical variation between the species of parasites as well as between prevalence of the same parasite in different áreas. Table 1 shows the percentages of the parasites found in all collected samples in each of the locations. The highest prevalence were found in the municipalities from the Northwest as well as some municipalities from the Southeast area of the studied region, because of the presence of E. aerophila, although in some of them, higher prevalence of Ancylostomidae gen. sp. and Strongyloides sp. were observed. The abundance of parasitization was also highly heterogeneous; with the highest egg count being by tapeworms (183 e/g feces) and the lowest by T. leonina (3 e/g feces). Furthermore, the highest parasite abundances were observed in the municipalities.
4. DISCUSSION
The coprological analysis helps to determine the presence of parasites in wild animals: In addition, these analysis helps to evaluate and understand the relationship between parasites and their hosts, providing information about their feeding habits and behavioral aspects [1].
In the present study, eggs and larvae of different species of parasitic helminths were observed. These parasites are transmitted through different transmission routes, mainly by the ingestion of embryonated eggs eliminated in feces from wolves or other parasitized mammals (Eucoleus spp., Trichuris spp.), penetration of infective larvae (L3) through the skin (Strongyloides sp., Ancylostomidae gen. sp.), eating an infected prey (Taenia spp.) or transplacental transmission (Toxocara spp. and Toxascaris spp.).
The prevalence and the abundance of parasitization were higher at the Northwest end of Sierra de la Culebra (Ancylostomidae gen. sp., Strongyloides sp., Eucoleus aerophilus and Trichuris vulpis). The high prevalence of Strongyloides sp. and Ancylostomidae gen. sp. may be due to the high humidity present in the Northwest of the studied area, which might favor the survival of infective larvae and eggs [1, 10]. Furthermore, the prevalence of these parasites was also high in the municipality of Tábara, in the Southeast; although climatic aspects may be implicated, it has not been possible to identify any conclusive factor. On the other hand, the number of analyzed samples in these municipalities was larger than in the other municipalities which may have influenced the results.
At present, there are no previous data on parasitosis by Strongyloides sp. in wolves in Spain and is similar in the neighboring country, Portugal, with 21.3% prevalence [14]. These analogous results are consistent considering the homogeneity present between the wolf populations from both countries and the similar climatic conditions. These results were confirmed by the Baermann method, in more than half of the samples, where it was possible to culture the larvae to differentiate them from other species of similar larvae. However, recently, 0.5% of larvae has been reported in Croatian wolves [15], so further studies are necessary to corroborate the observed data, taking into account the diversity of the results between the two studies.
The presence of Taenidae species was rare but rather homogeneous in those municipalities where these species were identified. Since these parasites are transmitted through the ingestion of meat contaminated with cysticerci (infective larvae) [12], it could be hypothesized that only highly predatory packs of wolves are affected. The low prevalence may be related to some other factors, such as poorly parasitized preys or wolves feeding mainly on peri-urban waste, as previously observed in other areas, possibly caused by the lack of suitable preys [14, 16-20]. The prevalence was lower than that reported by other authors in Spain, Taenia hydatigena been the most prevalent Taenidae (55%-58.6%) [1, 21]. The parasitic control on the cattle of the zone, which could be used as the food of the studied animals, can also be the cause of the low prevalence found in these animals. Studies carried out in the North of Europe showed a higher prevalence of Taenia spp. in Finland and Sweden (47%) but not in Poland (1.4%) [4, 22].
The prevalence of Ancylostomidae gen. sp. in this study was similar to that obtained by Domínguez and de la Torre [23] who observed 16.6% for Ancylostoma caninum and 11.1% for Uncinaria stenocephala. Other authors reported the presence of Ancylostomidae gen. sp. (Ancylostoma/Uncinaria) between 2% and 45.7% [22, 24]. Similar data have been published by other authors in Spain and Europe regarding the prevalence of Trichuris vulpis [25], except those from Balmori et al. [26] in the Cantabrian Mountains (42.9%). The prevalence of Eucoleus aerophilus was higher than others previously reported in Europe [8]. Essentially, Toxocara canis and Toxascaris leonina are directly transmitted from host to host by the interaction between wolves through the social behavior of the species. Also, these parasites can be transmitted by the ingestion of prey infected with larvae. The prevalence obtained for Toxocara canis was higher than those obtained by other authors in the Iberian Peninsula (3.4%-5%) [1, 21] and in Latvia (5.8%) [7]. In contrast, prevalence reported by Guberti et al. [3] in Italy was higher (17%). Furthermore, the prevalence of Toxascaris leonina described in this study was lower than that obtained in other studies conducted in Spain [1] and Estonia [6], but similar to that of Guberti et al. [3] in Italy (4%).
CONCLUSION
The data obtained shows a wide helminth fauna in the wolves from Sierra de la Culebra, similar to results reported in other studies carried out in Spain and European countries; the differences found in the abundance and distribution of parasites might be attributed to local environmental factors as well as animal behavior. Taeniidae gen. sp. was distributed more homogeneously from the territorial point of view, possibly due to similar feeding habits of the wolves. Finally, this study constitutes the first description of the presence of Strongyloides sp. in Canis lupus signatus in Spain.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethical committee of Veterinary Medicine Service of Las Palmas de Gran Canaria University.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank the collaboration of Forestry Rangers of Sierra de la Culebra Hunting Regional Reserve (Junta de Castilla y León), who contributed to collect samples.


