All published articles of this journal are available on ScienceDirect.
Vermamoeba vermiformis - A Free-Living Amoeba with Public Health and Environmental Health Significance
Abstract
Many case reports emphasize the fact that Free-Living Amoebae (FLA) can relatively easily get in contact with humans or animals. The presence of several facultative parasitic FLA in habitats related to human activities supports their public health relevance. While some strains of Acanthamoeba, Naegleria fowleri, Balamuthia mandrillaris and several other FLA have been described as facultative human pathogens, it remains controversial whether Vermamoeba vermiformis strains may have a pathogenic potential, or whether this FLA is just an incidental contaminant in a range of human cases. However, several cases support its role as a human parasite, either as the only etiological agent, or in combination with other pathogens. Additionally, a wide range of FLA is known as vectors of microorganisms (endocytobionts), hereby emphasizing their environmental significance. Among those FLA serving as hosts for and vectors of (pathogenic) endocytobionts, there are also descriptions of V. vermiformis as a vehicle and a reservoir of those endocytobionts. The involvement in animal and human health, the role as vector of pathogenic microorganisms and the pathogenicity in cell cultures, led to the assumption that V. vermiformis should be considered relevant in terms of public health and environmental health.
1. INTRODUCTION
Free-living amoebae (FLA) are predatory heterotrophic protozoa, feeding as trophozoites on bacteria, cyanobacteria, fungi and algae through phagocytosis while adhering to surfaces [1]. There are numerous case reports indicating how easily humans may get in contact with FLA. Some Acanthamoeba sp. strains, Naegleria fowleri, Balamuthia mandrillaris and several other FLA have proved to be facultative human pathogenic microorganisms [2].
The presence of pathogenic FLA in habitats related to human activities supports the public health relevance of these protozoa.
While those FLA genera or strains are well-known parasites of humans and animals, the discussion about the public health and environmental health relevance of V. vermiformis is still ongoing.
Vermamoeba vermiformis was described in 1967 by Page as Hartmannella vermiformis [3, 4]. Smirnov et al. and Page renamed this FLA as Vermamoeba vermiformis for both, morphological and phylogenetic reasons [4]. The genus Echinamoeba is a sister group of Vermamoeba [4-10]. V. vermiformis is widespread in nature and in artificial environments. It is an ubiquitous, cyst-forming FLA, showing a certain resistance (tenacity) in different environmental conditions [11, 12].
In this review study, the impact of V. vermiformis on humans and animals was analysed. An additional systematic literature search was done using databases such as PubMed, but also by screening conference papers of meetings dealing with FLA (e.g. such as the “International Meetings on the biology and pathology of free-living amoebae”). The key words used within the literature search were: “Vermamoeba vermiformis”, “Hartmannella vermiformis”. Those relevant publications dealing with V. (H.) vermiformis were included in this review.
2. MORPHOLOGY AND TAXONOMY
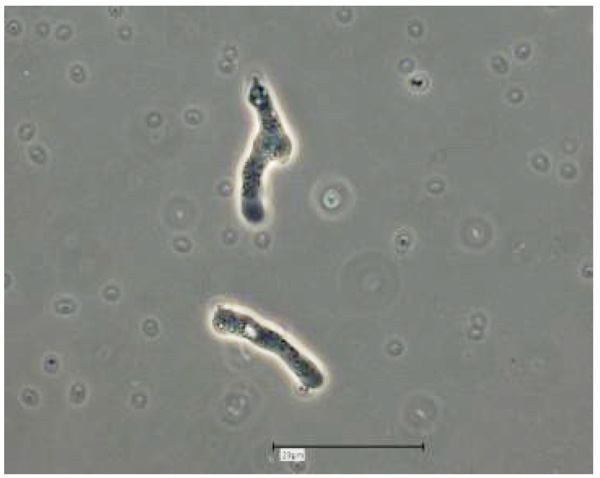
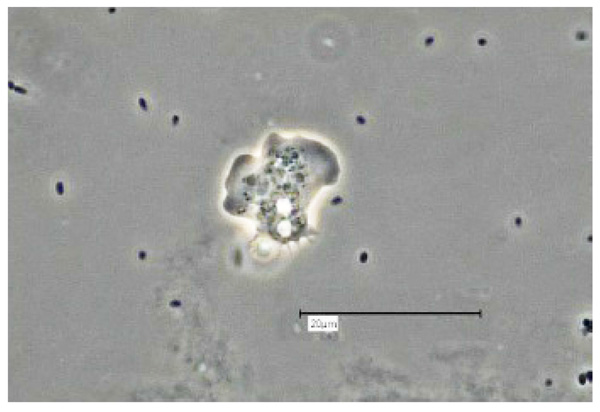
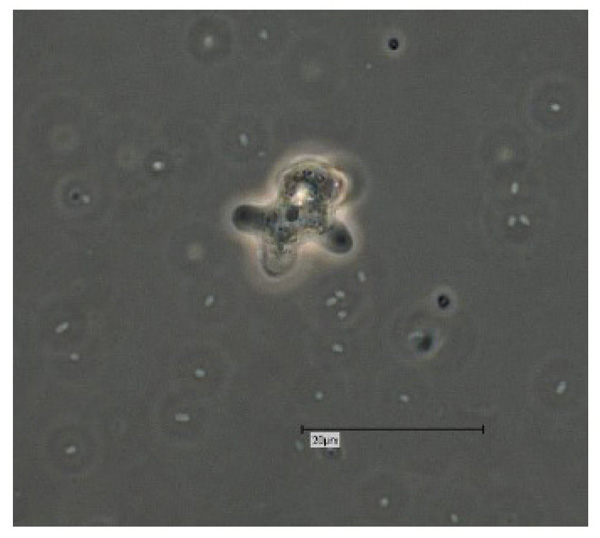
Vermamoeba vermiformis shows a two-stage life cycle with a trophozoite (division by binary fission, feeding, motility) and a dormant cyst form that enables V. vermiformis to survive in hostile environmental conditions such as nutrient depletion, osmotic stress, temperature changes, or pH variation [10]. V. vermiformis can excyst again as soon as the environmental conditions become favourable again. Page and Smirnov et al. (2011) provided morphological descriptions of the trophozoites and cysts of V. vermiformis [4, 13, 14]. These descriptions include morphological features (e.g. length, cylindrical trophozoites, branching with numerous pseudopodia under certain conditions, cyst diameter, and bilayer cyst walls; limax or slug-like motility). A flagellate form has not been described. The trophozoites of V. vermiformis show the typical worm-shaped or slug-like (elongated, cylindrical) morphology described by Page and Smirnov et al. [4, 13]. The locomotive forms are 22-42 μm long. Usually the trophozoites show a monopodial form (Fig. 1) in locomotion, some of them with uroidal filaments (Fig. 2). These uroidal filaments weren`t reported in early studies [3]. Granulae are visible within the cytoplasm as well as a small anterior hyaline zone [10, 15]. In general, morphological features seem to vary among the strains, determined by the environmental conditions. The trophozoites are usually monopodial. However, they may show several pseudopodia and irregular shapes, especially when moving in another direction (Fig. 3).
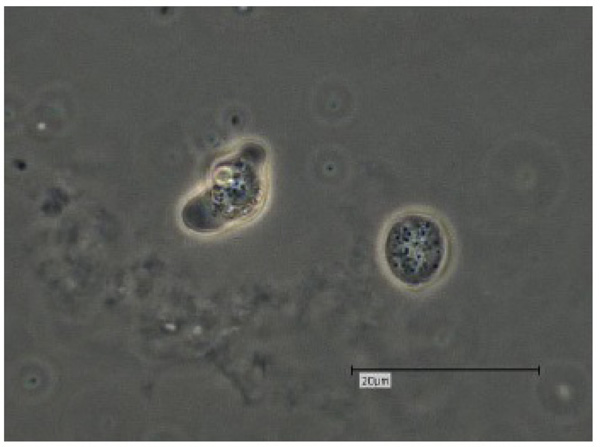
The V. vermiformis cysts are of spherical, round shape with a diameter of 6-9μm. The cyst consists of a 50 nm thick endocyst and a 110 - 140nm thick ectocyst. The two-layered cyst wall (double-wall) contains proteins and glucose polymers. The cyst shows one or two nuclei; no pores or ostioles are visible (Fig. 4) [16]. This cyst stage protects V. vermiformis against hostile environmental conditions such as nutrient depletion, osmotic stress, temperature changes, or pH variation. Fouque et al. (2012) showed that the encystment process of V. vermiformis lasts about 9 hours [11].
V. vermiformis belongs taxonomically to the class Echinamoebida, phylum Tubulinea [4-9].
The phylogenetic discrepancy and the detected morphological differences from the other Hartmannella spp. has led to the introduction of the new genus Vermamoeba, with the only species V. vermiformis. As there is a certain variability in trophozoite morphology and a morphological similarity to other FLA cysts, alternative methods have to be used for accurate detection, including molecular techniques.
Molecular-based analyses of V. vermiformis are limited to sequencing of the 18S rRNA gene, which is used as a phylogenetic marker for taxonomic inferences [10]. Those 18S rRNA sequences, publicly available, show a high degree of sequence conservation for V. vermiformis. This leads to the assumption that the 18S rRNA gene sequences may be insufficient to compare those strains of V. vermifomis with different virulence patterns.
3. EPIDEMIOLOGY
3.1. Habitats of Vermamoeba vermiformis
Natural freshwater environments, surface water, soil and biofilms are the natural habitats for V. vermiformis. It is one of the most prevalent FLA found in humid ecosystems, other than Acanthamoeba spp [17, 18]. Within its biocenosis, V. vermiformis contributes to microbial communities in biofilms while feeding on bacteria. It has been detected in waters, springs, snow, and soil [19-21].
In addition to these natural humid habitats, V. vermiformis has been isolated from man-made environments and engineered water systems such as tap water, fountains, water pipes, bottled mineral water, drinking water sources, recreational waters and swimming pools [10, 11, 19, 22-28].
In several studies, V. vermiformis has been found within the human environment, showing paradigmatically how easily humans may find themselves in close contact with these FLA. V. vermiformis has been detected in composts, aerosols from composting facilities, wastewater from the textile industry [10, 28, 29], contact lens storage cases, and even hospital ward dust and biofilms [24, 30-33].
V. vermiformis has been found in thermal springs with temperatures of 41-53 °C and a pH of 4.9-7 [27, 34-36]. Rohr et al. identified V. (resp. Hartmannella) vermiformis in 65% of the hot water samples which were positive for amoebae and on 15% of swabs from moist areas [37, 38].
3.2. Vermamoeba vermiformis as a Parasite for Humans
The role of V. vermiformis as a potential pathogenic parasite of humans has been discussed for many years. Some authors have concluded that V. vermiformis is an environmental contaminant without any involvement in pathogenesis [39-41].
A certain cytopathogenicity of the thermo-tolerant V. vermiformis on keratocytes was confirmed in in vitro studies [41, 42]. Another hint regarding the (veterinary medical) pathogenic potential is the fact that some strains of V. (Hartmannella) vermiformis have produced tissue lesions in experimentally infected fish [15]. At least, pathogenicity of V. vermiformis for aquatic organisms cannot be excluded.
The medical significance of V. vermiformis (resp. Hartmannella vermiformis) and especially its pathogenic potential as a parasite for humans has been reported from several countries, including Spain, Costa Rica, Peru, France, Scotland, Japan and Iran [16, 43, 46-49]. The isolation of V. vermiformis from humans or while examining human infections, has provided evidence of its potential involvement in the pathogenesis. In 2014, V. vermiformis was detected in human nasal swabs [16]. Most of the “case reports” with an involvement of V. vermiformis have included corneal damage [10]. A keratitis case included a female contact lens wearer, who presented initially with eye pain, redness, blurred vision, photosensitivity, tearing, and a sensation of a foreign body in her eye [45]. In other cases V. vermiformis was isolated while examining for an Acanthamoeba keratitis [50-53]. Interestingly, V. vermiformis has been found predominantly in mixed amoebic keratitis cases [46, 50, 51, 53, 54]. These findings, together with the results of other studies, have led to the conclusion that the spectrum of protozoa contaminating contact lens solutions is broader than previously known and includes Acanthamoeba spp., Balamuthia mandrillaris and also V. vermiformis [44, 50-53].
Two human cases involving V. vermiformis have been recently examined in Germany: V. vermiformis was detected within the contact lens cases of a bacterial keratitis patient. In this “case report” V. vermiformis seemed to be a contaminant without (significant) involvement in the pathogenesis.
A second case included the only non-keratitis case report so far - with an exclusive isolation of V. vermiformis from a human. V. vermiformis has been confirmed as potential etiological agent in a 27 years old female patient, who presented with a weeping wound developing as a painful ulcer on the upper eyelid at the medial angle of the right eye. Cultivation, morphological microscopical analysis and nucleic acid techniques, followed by sequence analysis revealed V. vermiformis as the only detected microorganism involved in this case. The confirmed presence of V. vermiformis in the ulcer and the proven absence of the typical pathogenic bacterial microorganisms led to the strong assumption that V. vermiformis may be involved in the pathogenesis of this human case.
3.3. Vermamoeba vermiformis as Vector of Endocytobionts
In general, naked free-living amoebae (FLA) graze in biofilms and feed on bacteria, algae, yeasts and other protozoa. They capture their prey by phagocytosis following chemotactic trophism and transfer them to lysosomal compartments in the phagocytic pathway where they are usually digested by enzymes [1]. However, some of these intracellular microorganisms (endocytobionts) have developed a strategy to avoid lysis and digestion during the phagocytic process. The impact of such interactions of FLA and their endocytobionts with respect to Public Health and Environmental Health was described recently [54, 55]. Additionally, a wide range of FLA is known to be vectors of endocytobionts [2].
The relevance of the FLA - endocytobiont relationship in terms of pathogenicity, tenacity, virulence enhancement, protection, gene transfer etc. is the focus of current research [56].
While there is still an ongoing discussion about the public health relevance of V. vermiformis as a human parasite, it has proven to be a vector of endocytobionts, some of these being confirmed pathogens of public health significance [for overview see: 10 or 57]. These endocytobionts may also play a significant role in aggravating the infection or in enhancing inflammatory processes [14, 58].
In 1988 V. (Hartmannella) vermiformis was described as an important protozoon in the ecology of Legionella pneumophila [59]. Since then V. vermiformis is known as a reservoir and vector of Legionellae [17, 60-64].
V. vermiformis also has proven to serve as a vehicle for and vector of Bacillus anthracis, Neochlamydia hartmannellae and other Chlamydia-like endocytobionts [65-67; for overview see: 10, 57 or 60]. Other bacterial microorganisms persisting as endocytobionts and replicating intracellularly in V. vermiformis are Protochlamydia massiliensis, Protochlamydia phocaeensis and Rubidus massiliensis [68-70].
V. vermiformis may also be associated with Mycobacteriae. in vitro studies revealed that highly pathogenic Mycobacterium leprae remained viable and virulent within V. vermiformis cysts [10, 71, 79]. Even on nasal swabs V. vermiformis and Mycobacterium chelonae were detected sympatrically [16].
V. vermiformis can be permissive for Pseudomonas aeruginosa [72]. There are also interesting relationships of V. vermiformis with Stenotrophomonas maltophilia (an important nosocomial pathogen) and Campylobacter jejuni [19, 33, 73-75].
Francisella novicida has proven to be another endocytobiont of V. vermiformis, surviving and replicating intracellularly in non-acidified phagosomes [76].
An intranuclear endocytobiont of V. vermiformis has been described recently and named Nucleicultrix amoebiphila [77].
As V. vermiformis is isolated frequently in environmental samples, it has been used as a potential host in the search for giant viruses (giruses). Some of those giruses were detected in V. vermiformis: Several isolates of Faustovirus, an Asfarvirus-related lineage of giruses, were isolated using V. vermiformis as the host amoeba [78]. Kaumoebavirus and Orpheovirus are further examples of giruses proliferating in V. vermiformis [79, 80].
Interactions between V. vermiformis and fungi include Exophiala dermatitidis, Aspergillus fumigatus, Candida spp. and Fusarium oxysporum [14, 81-84].
In the context of acting as vector of pathogenic endocytobionts, V. vermiformis may also play an important role in the distribution of food borne microorganisms. Several species of FLA have been discovered on vegetables, green salad, spinach, lettuce, as well as in chicken coops and refrigerators. Studies to determine the abundance of free-living protozoa on dishcloths as a possible source for cross-contamination in food processing establishments have been conducted recently. Vahlkampfia, Vannella, Acanthamoeba, Hyperamoeba, and Vermamoeba have been detected on these dishcloths [53, 55].
CONCLUSION
V. vermiformis is certainly another FLA to be considered as a potential parasite of humans or animals and as a vector of encocytobionts. The case reports with an involvement of V. vermiformis and especially one of the recent cases with V. vermiformis as the only detected microorganism, indicate that this FLA may be potentially (or opportunistically) pathogenic. The thermo-tolerant V. vermiformis should definitely be considered in future studies and/or diagnostics targeting FLA as etiological agents of human and animal diseases. When comparing the studies of V. vermiformis as a potential parasite for humans and its role as vector of potentially pathogenic microorganisms, we must (still) come to the conclusion that V. vermifomis is more important as a vector, according to the literature available at present.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author would like to thank Dr. David Lam (MD, MPH, Shaman Medical Consulting) for review and English language editing of the article and Dr. C. Balczun for fruitful discussions.


