Susceptibility Status of Aedes aegypti L. Against Different Classes of Insecticides in New Delhi, India to Formulate Mosquito Control Strategy in Fields
Abstract
Background:
Mosquito control is a major concern throughout the world because of rising cases of mosquito-borne diseases. The outbreak of Zika, Dengue and Chikungunya has caused grave situations raising urgent need to control Aedes aegypti. Moreover, extensive use of synthetic insecticides in mosquito control programs has resulted in high levels of insecticide resistance leading to the use of magnified concentrations, impacting human health and environment adversely. The knowledge about current status of the insecticide susceptibility against Ae. aegypti could help to devise mosquito control strategy.
Objective:
Present study evaluates the larvicidal potential of thirteen insecticides belonging to seven different classes; organochlorines, organophosphates, carbamates, pyrethroids, neonicotinoids, avermectins and secondary metabolites; against early fourth instars of Ae. aegypti.
Materials and Methods:
The insecticide susceptibility was evaluated as per WHO protocol. Fatality counts were made after 24h of exposure; and the LC50, LC90 and other statistical parameters were computed by probit-regression analysis.
Results:
The data reveals the maximum efficacy of pyrethroids and fenitrothion, with lethal values less than 0.001 ppm. Avermectins, organochlorines and carbamates were moderately toxic, while neonicotinoid posed appreciable toxicity. In contrast, berberine, a secondary plant metabolite was found inefficient. The larvicidal efficacy of tested insecticides against Ae. aegypti was found in the decreasing order of pyrethroids > organophosphates > avermectins > organochlorines > carbamates > neonicotinoids > secondary metabolites.
Conclusion:
Present investigations explore various toxicants as Dengue vector control agents in order to devise a suitable control strategy for mosquito control in fields.
1. INTRODUCTION
Mosquito-borne diseases; prevalent in more than 100 countries across the world, infecting over millions of individuals every year at the global level; are among the leading cause of human deaths [1, 2]. Aedes aegypti L. is today considered the most threatening vector in the tropics creating a havoc with a range of diseases like Dengue, Yellow fever, Chikungunya and Zika. As per WHO report, currently 47 countries are at the risk of severe Dengue while 60 countries are under the attack of Chikungunya [3]. In recent decades, the incidences of Dengue have grown dramatically around the world. Moreover, under-reporting of actual numbers of Dengue cases and their misclassification has made the disease serious and uncontrollable. According to the recent estimates, human population experiences 390 million Dengue infections per year (95% credible interval 284-528 million), of which 96 million manifests clinically [4, 5].
Dengue contagion is one of the most probative Aedes-borne viral diseases of humans in tropics. In India, diseases transmitted by Ae. aegypti and Ae. albopictus have shown a significant rise during the last decade. According to the data compiled by the Ministry of Family and Health Welfare, India experienced a total of 1,29,166 cases of Dengue with 245 fatalities in 2016 which substantially increased to 1,53,635 cases in 2017 leading to 226 deaths [6]. In addition, with the recent outbreak of Chikungunya across India with a total of 62,268 cases in 2017, Ae aegypti has taken a huge attention of researchers [6]. Moreover, diseases like Zika are on the rise causing grave situation. Keeping in view the lack of an adequate and successful vaccine against these diseases, control of mosquito vector by large scale larval mortality is the only solution [7].
The most recommended plan to control mosquito-borne diseases primarily lies on mosquito management below threshold level and interrupting their disease-transmission cycle. Various control measures; elimination of their breeding places, use of several biological agents, sterile insect release method, etc.; have been devised and practiced till date [8]. Insecticides belonging to different groups, especially DDT (organochlorine) and malathion (organophosphate) have been in extensive use for the past few decades in vector control programmes in India. Though majorly all the organochlorines are banned by EPA for residential usage due to acute toxicity, they are still in use in agricultural fields [9].
Exploration of new insecticides led to the discovery and application of organophosphates and carbamates in pest control. Gradually, they were also banned by US-EPA as part of the food and quality protection act due to reports of ground water contamination in California [10]. Malathion used extensively in agriculture, residential landscaping and in eradication of mosquitoes was replaced by novel pyrethroids due to adverse environmental impact [11]. Subsequently, synthetic pyrethroids were employed for Indoor Residual Spraying (IRS) in areas with multiple insecticide-resistant vectors, for the treatment of mosquito nets (Insecticide-Treated Nets or ITNs) and then for manufacturing of long-lasting insecticidal nets (LLINs) [12-14].
Pyrethroids, synthetic analogs of pyrethrins isolated from the flowers of Chrysanthemum have been labeled as safe by WHO for use in vector control programs [15-17]. However, continued and random use of pyrethroids has resulted in the development of resistance in mosquito vectors and threat to aquatic life [18, 19]. Consequently, new insecticides were explored and pyrethroids were replaced by neonicotinoids and other compounds of novel chemistry. Till date, no report of high levels of neonicotinoid resistance and cross-resistance against them has been cited in literature [20]. In general, neonicotinoids are found to be of very low toxicity to mammals and non-target organisms, rendering them as efficient alternates for mosquito management [21]. Nowadays, avermectins are also being explored as broad-spectrum pesticides reported to possess efficacy against a variety of arthropods [22] and nematodes [23, 24].
Devising a suitable mosquito management program requires the latest reports on the susceptibility status of that particular species against different insecticides in use. As it is well known that injudicious pesticide applications against that insect pest often leads to environment pollution and harmful effects on human and non-target species; it becomes imperative to evaluate the toxicities of different insecticides to formulate control strategies.
Lack of baseline susceptibility data, continued rise in Aedes-borne diseases and increase in the insecticide resistance in Ae. aegypti, has forced us to assess the current insecticide susceptibility status in the larvae of Ae. aegypti. Present investigations, thus attempts to take a comprehensive view of susceptibility in larvae of Dengue fever vector against OCls, OPs, Carbamates, Synthetic pyrethroids, Neonicotinoids, Avermectins and Secondary metabolites currently utilized against them. The study will provide a better understanding and will give a clear picture about the susceptibility of Ae. aegypti towards both traditionally used and newly introduced insecticides. It will help to devise a suitable mosquito control strategy which is of prime importance nowadays.
2. MATERIALS AND METHODS
2.1. Culture of Mosquitoes
Dengue fever mosquitoes, Ae. aegypti, were collected from different areas of New Delhi (Fig. 1). The colony of Ae. aegypti was maintained in an insect rearing laboratory under controlled conditions of 28± 1° C, 80 ± 5% RH, 14 h of light and 10 h of darkness [25]. Adults kept in clothed cages were fed on sugary juice by supplying them raisins soaked in water. Female mosquitos were provided with occasional blood meals for egg maturation by keeping albino rat in the cage. The eggs were collected in an ovitrap lined with Whatman filter paper strips which were then transferred into the enamel trays filled with at least 1.5-2.0 L of dechlorinated water. The hatched larvae were fed on powdered dog biscuits and yeast in a ratio of 3:1 [26]. The pupae were collected on regular basis and were kept in clothed cages for adult emergence.

 Pitampura [Latitude, Longitude (28.705493, 77.132341) 28°42′19.8′′N and 77°07′56.4′′E];
Pitampura [Latitude, Longitude (28.705493, 77.132341) 28°42′19.8′′N and 77°07′56.4′′E];  Shahdara [Latitude, Longitude (28.700001, 77.291662) 28°42′00.0′′N and 77°17′30.0′′E];
Shahdara [Latitude, Longitude (28.700001, 77.291662) 28°42′00.0′′N and 77°17′30.0′′E];  Saket [Latitude, Longitude (28.521625, 77.213702) 28°31′17.9′′N and 77°12′49.3′′E];
Saket [Latitude, Longitude (28.521625, 77.213702) 28°31′17.9′′N and 77°12′49.3′′E];  Sarita Vihar [Latitude, Longitude (28.531703, 77.288247) 28°13′54.1′′N and 77°17′17.7′′E];
Sarita Vihar [Latitude, Longitude (28.531703, 77.288247) 28°13′54.1′′N and 77°17′17.7′′E];  Lodhi Colony [Latitude, Longitude (28.579848, 77.215736) 28°34′47.5′′N and 77°12′56.7′′E]
Lodhi Colony [Latitude, Longitude (28.579848, 77.215736) 28°34′47.5′′N and 77°12′56.7′′E]
2.2. Preparation of Insecticidal Solutions
The susceptibility of Ae. aegypti larvae were conducted against seven groups of insecticides. The details of thirteen insecticides investigated are presented in Table 1. Among these; Organochlorines (lindane, endosulphan), Organophosphates (malathion, fenitrothion) and Pyrethroids (deltamethrin, alpha-cypermethrin, lambda-cyhalothrin, permethrin) were procured from M/s Aimco Insecticides, Mumbai India; whereas rest of the insecticides; acetamiprid, carbofuran, fenobucarb, emamectin benzoate and berberine were obtained from M/s Sigma Aldrich.
| Insecticide | IUPAC name | Structure | Mode of action | Percent purity |
|---|---|---|---|---|
| Organochlorines | ||||
| Lindane | (1r,2R,3S,4r,5R,6S)-1,2,3,4,5,6-hexachlorocyclohexane | 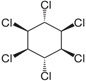 |
Inhibit gamma-Aminobutyric acid (GABA) chloride ionophore complex | 99.5 |
| Endosulphan | 6,7,8,9,10-Hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepine-3-oxide | 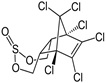 |
Inhibit gamma-Aminobutyric acid (GABA) chloride ionophore complex | 96.5 |
| Organophosphates | ||||
| Malathion | Diethyl 2-[(dimethoxyphosphorothioyl) sulfanyl] butanede | 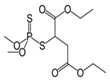 |
Inhibit acetylcholine esterase enzyme | 95.0 |
| Fenitrothion | O,O-Dimethyl O-(3-methyl-4-nitrophenyl) phosphorothioatek | 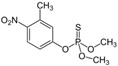 |
Inhibit acetylcholine esterase enzyme | 96.7 |
| Carbamates | ||||
| Carbofuran | 2,2-Dimethyl-2,3-dihydro-1-benzofuran-7-yl methylcarbamate | 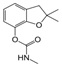 |
Inhibit acetylcholine esterase enzyme | 98.0 |
| Fenobucarb | 2-Butan-2-ylphenyl) N-methylcarbamate | 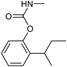 |
Inhibit acetylcholine esterase enzyme | 95.0 |
| Pyrethroids | ||||
| Lambda-cyhalothrin | 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethyl-cyano(3-phenoxyphenyl) methyl cyclopropanecarboxylate | 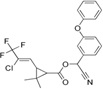 |
Modulate sodium channels | 98.0 |
| Alpha-cypermethrin | [Cyano-(3-phenoxyphenyl) methyl] 3-(2,2-dichloropropane-1-carboxylate | 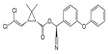 |
Modulate sodium channels | 97.0 |
| Permethrin | (±)-3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate | 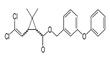 |
Modulate sodium channels | 94.0 |
| Deltamethrin | [(S)- Cyano-(3-phenoxyphenyl)-methyl] (1R,3R)-3-(2,2-dibromoethenyl)-2,2-dimethyl-cyclopropane-1-carboxylate | 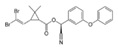 |
Modulate sodium channels | 98.0 |
| Neonicotinoid | ||||
| Acetamiprid | N-[(6- chloro-3-pyridyl) methyl]-N’-cyano-N-methyl-acetamidine | 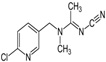 |
Compete with nicotinic acetylcholine receptors | 99.9 |
| Alkaloid-Secondary metabolite | ||||
| Berberine | 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium | 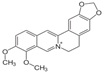 |
Singlet oxygen generation effect DNA Pol-I | 95.0 |
| Avermectin | ||||
| Emamectin benzoate | 4′′-Deoxy-4′′-epi-methylamino-avermectin (Medical Subject Heading -MeSH) |
 |
Modulate glutamate gated chloride channels (allosteric modulators) | 99.3 |
All the insecticides were diluted in ethanol (eMerck) and desired stock concentrations were prepared. Each solution was stored at 4°C and assessed for their efficacy against larvae of Dengue fever vector.
2.3. Larvicidal Bioassays with Insecticides Against Aedes aegypti
The larvicidal efficacy of the insecticides against Ae. aegypti early fourth instars were assessed as per WHO protocol [25] with few modifications. The graded series of each insecticide was prepared to conduct the bioassay. For each bioassay, a total of 25 early fourth instar larvae of Ae. aegypti were taken in a bowl filled with 89 mL of dechlorinated water. These were then transferred to glass beakers containing 110 mL of dechlorinated water and 1 mL of the particular concentration of an insecticide [27, 11]. For each concentration, three replicates were run simultaneously making a total of 75 larvae for each concentration of a particular insecticide.
The dead and moribund larvae were counted and recorded after 24 h of exposure. The larvae were touch with a glass rod to assess their survival status. Control experiments were run simultaneously by substituting insecticidal solution with ethanol.
2.4. Data Analysis
The bioassays resulting in more than 20% larval mortality or pupae formation in control indicated the inappropriate selection of larvae and thus were discarded and run again. However, if 5-20% larval mortality was obtained in control assays, it was corrected by Abbott’s formula [28].
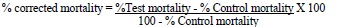 |
The concentration-response data obtained and recorded was then analyzed by probit-regression analysis using a statistical software program SPSS 19.0. The median lethal concentrations (LC50) values, 90% lethal concentration values (LC90), their 95% Confidence Intervals (CI), slope, Standard Error (SE), Regression Coefficient (RC)/Slope and chi square values were calculated. The LC50 values were considered significantly different when their 95% CI did not overlap.
3. RESULTS
The larvicidal potentialities of thirteen insecticides belonging to different classes - Organochlorines (Malathion, Fenitrothion), Organophosphates (Endosulphan, Lindane), Carbamates (Carbofuran, Fenobucarb), Pyrethroids (Deltamethrin, Lambda-cyhalothrin, Alpha-cypermethrin, Permethrin), Neonicotinoid (Acetamiprid), Avermectin (Emamectin benzoate) and Secondary metabolite (Berberine) - evaluated against early fourth instar of Ae. aegypti L are presented in Tables 2 to 6. The results obtained clearly show the maximum efficacy of alpha-cypermethrin among all the insecticides investigated revealing lowest LC50 value of 0.00048 ppm. Among the two organochlorines tested, lindane exhibited five times higher toxicity than endosulphan which caused 50% larval mortality at 0.39 ppm (Table 2). Similarly, among the OPs, fenitrothion was found to be highly toxic while malathion showed moderate toxicity (Table 3). It is also evident from the data that Ae. aegypti early fourth instars exhibited more susceptibility towards OPs as compared to OCls.
| S. No. | Insecticides | LC50 in ppm | LC90 in ppm | χ2 (df) |
Slope ± SE |
|---|---|---|---|---|---|
| 1 | Lindane | 0.0832 (0.0662-0.1014) |
0.4011 (0.3062-0.5819) |
5.29 (7) | 1.737±0.28 |
| 2 | Endosulphan | 0.3987 (0.2863-0.5085) |
0.7163 (0.5552-1.1754) |
9.64 (5) | 1.29±0.65 |
| S. No. | Insecticides | LC50 in ppm | LC90 in ppm | χ2 (df) |
Slope ± SE |
|---|---|---|---|---|---|
| 1 | Malathion | 0.0548 (0.0472-0.0620) |
0.1058 (0.0930-0.1252) |
1.735 (4) | 4.5369±0.72 |
| 2 | Fenitrothion | 0.00063 (0.0003-0.0013) |
0.00392 (0.0016-0.3509) |
5.084 (4) | 0.8086±0.36 |
The larvicidal bioassay with carbamates against Dengue vector revealed less efficacy as compared to OCls and OPs. Moreover, both the carbamates used; carbofuran and fenobucarb, were found almost equally effective resulting in respective LC50 value of 0.52 ppm to 0.59 ppm (Table 4).
| S. No. | Insecticides | LC50 in ppm | LC90 in ppm | χ2 (df) |
Slope ± SE |
|---|---|---|---|---|---|
| 1 | Carbofuran | 0.5253 (0.4642-0.5888) |
1.0576 (0.9006-1.3521) |
5.888 (3) |
4.0612±0.55 |
| 2 | Fenobucarb | 0.5914 (0.5241-0.6510) |
1.0861 (0.9763-1.2537) |
3.575 (5) |
4.3071±0.76 |
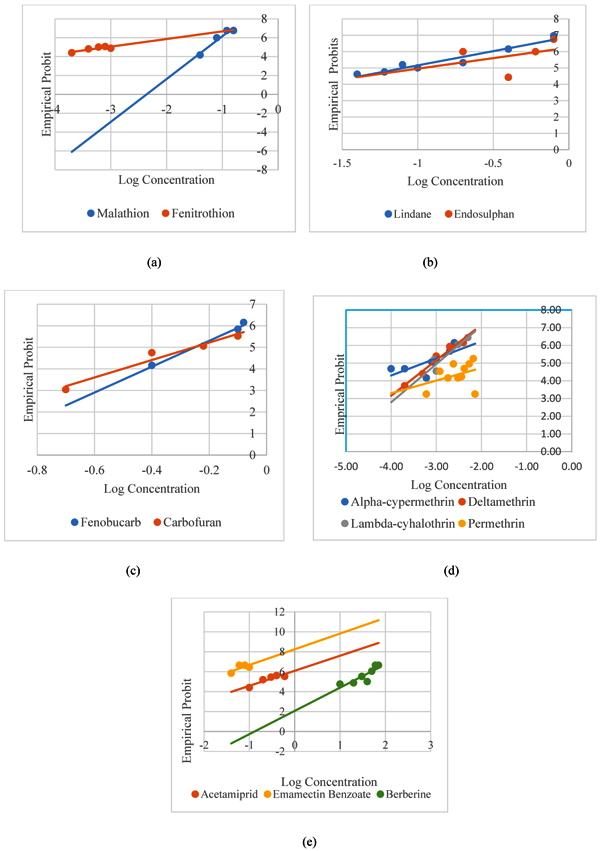
In the present study, four pyrethroids were also investigated for their efficiency against larvae of Dengue fever vector. The larvae showed high susceptibility towards pyrethroids as compared to rest of the investigated classes of insecticides. The results revealed that permethrin was least toxic out of the four pyrethroids possessing LC50 of 0.007 ppm. The alpha-cypermethrin, which exhibited comparable efficacy to fenitrothion, was observed to be 2-fold more effective than deltamethrin and lambda-cyhalothrin whereas it showed 13-fold more larvicidal efficacy than permethrin. Moreover, the LC90 values of three pyrethroids; deltamethrin, lambda-cyhalothrin and alpha-cypermethrin were found to be overlapping except permethrin which showed an LC90 of 0.1 ppm (Table 5 and Fig. 2).
| S. No. | Insecticides | LC50 in ppm | LC90 in ppm | χ2 (df) |
Slope ± SE |
|---|---|---|---|---|---|
| 1 | Deltamethrin | 0.0008 (0.0006-0.0010) |
0.0034 (0.0025-0.0054) |
1.615 (4) | 2.003±0.35 |
| 2 | Lambda-cyhalothrin | 0.0009 (0.0004-0.0014) |
0.0035 (0.0022-0.0109) |
6.458 (5) | 2.174±0.39 |
| 3 | Alpha-cypermethrin | 0.0005 (0.0002-0.0009) |
0.0037 (0.0016-0.0450) |
23.175 (8) | 0.966±0.20 |
| 4 | Permethrin | 0.0074 (0.0050-0.0150) |
0.0817 (0.0281-1.5081) |
13.372 (8) |
1.5145±0.28 |
Bioassays with neonicotinoids evidenced their considerable efficacy against early fourth instars of Ae. aegypti. Acetamiprid showed a moderate LC50 and LC90 value of 0.1879 ppm and 1.3155 ppm (Table 6). Emamectin benzoate, an avermectin was found to be 14 times more effective than acetamiprid against larvae of Ae. aegypti exhibiting LC50 of 0.013 ppm and LC90 of 0.060 ppm. Berberine, an alkaloid belonging to secondary metabolite, which is an active component inplant like Argemone mexicana, when assayed for larvicidal potential did not show appreciable toxicity revealing LC50 of 20.24 ppm and LC90 of 62.60 ppm (Table 6).
| S. No. | Insecticides | LC50 in ppm | LC90 in ppm | χ2 (df) |
Slope ± SE |
|---|---|---|---|---|---|
| 1 | Acetamiprid | 0.1879 (0.1193-0.2477) |
1.3154 (0.7586-5.2407) |
1.698 (3) | 1.516±0.49 |
| 2 | Berberine | 20.2432 (11.1741-29.3252) |
62.6057 (41.4218-156.8984) |
17.044 (7) |
2.339±0.35 |
| 3 | Emamectin benzoate | 0.0128 (0.00001-0.0294) |
0.05928 (0.0315-0.0992) |
0.741 (3) |
1.569±1.11 |
Comparative toxicity of all the thirteen insecticides used in the present study revealed that exposure to 2 ppm of all the insecticides caused 100% larval mortality except berberine which was found ineffective. (Fig. 3).

4. DISCUSSION
Different classes of insecticides; such as pyrethroids, organophosphate, organochlorines, carbamates have been used from decades to control mosquitoes. The extensive use of these insecticides has caused the problem of resistance in mosquito vectors. Thus, evaluation of susceptibility of different synthetic insecticides for selection of the most efficacious compound is an important step for devising strategy for effective mosquito control.
In the present study an attempt was made to assess the larvicidal activity of 13 different insecticides belonging to 7 different classes; namely Pyrethroids (Deltamethrin, Alpha-Cypermethrin, Lambda-Cyhalothrin and Permethrin), Organophosphates (Malathion and Fenitrothion), Organochlorines (Endosulphan and Lindane), Carbamates (Carbofuran and Fenobucarb), Neonicotinoid (Acetamiprid), Bioactive plant metabolite (Berberine) and Avermectin (Emamectin benzoate).
Our results showed that alpha-cypermethrin exhibited maximum toxicity against early fourth instars of Ae. aegypti as compared to other pyrethroids as well as other groups of insecticides which indicates it to be the most promising agent to be incorporated in the integrated vector management programmes. It is well known that pyrethroids are the sodium channel modulators that are being extensively used for the control of mosquitoes. Further, they are highly recommended by WHO for mosquito management due to their low mammalian toxicity and environmental safety [17]. Earlier, Kumar et al. [18] revealed much lower LC50 value (0.000118 ppm) when larvae of Dengue fever vector were assayed with deltamethrin. They also reported development of 703-fold deltamethrin resistance after continuous exposure till 40 successive [18]. A study conducted in Cochin found that Ae. aegypti and Ae. albopictus were resistant to DDT and dieldrin, but susceptible to propoxur, fenitrothion, malathion, deltamethrin, permethrin and lambda-cyhalothrin [29]. The investigations in Saudi Arabia reported comparable results when lambda-cyhalothrin was assayed against Ae. aegypti larvae resulting in LC50 of 0.007 ppm [30]. In Nigeria, larval bioassays carried out against IV instar larvae of Ae. aegypti and Cx. quinquefasciatus with dieldrin, dichlorvos and permethrin resulted in respective LC50 values of 0.48, 37.09 and 0.29 µg/L and 0.11, 10.05 and 0.05 µg/L [31] clearly indicating higher efficacy of pyrethroids as compared to other groups of insecticides.
Our results showed comparatively less toxicity of deltamethrin and lambda-cyhalothrin among all four tested pyrethroids though they were found to be more effective than rest of the insecticides tested except fenitrothion. The reduced susceptibility to these pyrethroids may possibly be linked with its extensive usage at broader scale for the control of Dengue fever vectors [18]. Our results suggest to use alternative pyrethroids for effective control of Ae. aegypti. The present bioassays also showed relatively low efficacy of organochlorines as compared to pyrethroids and organophosphates, but showed higher cidal value with respect to carbamates. The lindane was found to be five times more toxic than endosulphan. These insecticides have been in use since decades for control of various insect pests. Consequently, mosquitoes seem to have develop resistance against them making them less effective. Also, similar mode of action of organochlorines and pyrethroids often results in cross-resistance and is the possible cause of gradually reduced susceptibility to these insecticides.
Our investigations also showed much higher larval toxicity of fenitrothion as compared to another organophosphate, malathion; organochlorines, carbamates and most of the pyrethroids. The low toxicity of malathion may be attributed to its widespread application in mosquito management programmes since decades [29]. These results recommend to utilize alternate insecticides against which larvae of Dengue fever vector are susceptible. In Western Venezuela; the larvicidal activity of malathion was found in range of 1.6 ppm to 3.49 ppm against Ae. aegypti; much higher than obtained by us [32].
The neonicotinoids function as the nicotinic acetylcholinesterase inhibitors in the insect nervous system [21]. Acetamiprid has been reported to be moderately toxic against invertebrates and nontoxic to non-target organism [33]. Our results showed moderate toxicity of acetamiprid resulting in LC50 and LC90 values of 0.188 ppm and 1.315 ppm against early fourth instars of Ae. aegypti. Comparatively low toxicity of acetamiprid could be linked with its widespread usage in the management programs of bed bugs and the other insect pests [34]. The evaluation of the efficacy of different neonicotinoids against three different strains of mosquitoes revealed efficacy of imidacloprid against Ae. aegypti with much higher lethal values; LC50 and LC90 as 0.558 ppm and 6.135 ppm [35].
In case of other relatively new insecticides, emamectin benzoate, a GABA receptors stimulator causing increase in membrane chloride ion permeability was found to exhibit high toxicity against Ae. aegypti larvae rendering it a suitable option in the Dengue vector management. It resulted in higher larval mortality at lower dosages than caused by carbamates, endosulfan, acetamiprid and berberine. However, it extended much less toxicity when compared to that of pyrethroids. James et al. [36] had examined the efficacy of 3 different avermectins against both susceptible and insecticide-resistant laboratory strains of blowfly, Lucilia cuprina. All 3 compounds were found more toxic to blowfly larvae as compared to the OP compound, diazinon. Moreover, no significant cross-resistance to avermectins has been reported in houseflies resistant to dieldrin, DDT, diazinon or permethrin making it an efficient option [37].
The range of response to various insecticides in Ae. aegypti larvae could represent the natural variation between essentially susceptible populations. Consequently, the information presented here may be used as baseline data to monitor development of resistance in mosquitoes. When berberine, an alkaloid present in variety of plants like Argemone mexicana was tested against the larvae of Dengue fever vecto, it was found quite ineffective. The assays revealed 62 ppm as LC90 value which was quite high as compared to the results obtained by Philogène et al. [38]. They also showed that mosquito larvae that were treated with 10 ppm berberine plus near UV for 24 hours, exhibited the LC50 of 8.8 ppm compared to 250 ppm for dark controls.
Insecticide resistance has been a problem in all insect groups that serve as vectors of emerging diseases. Although mechanisms by which insecticides become less effective are similar across all vector taxa, each resistance problem is potentially unique and may involve a complex pattern of resistance foci. The main defense against resistance is close surveillance of the susceptibility of vector populations [39]. It is generally believed that employing an insecticide with highest efficacy would give best results in pest management programmes. However, the long-term and continued utilization of the only one insecticide could result in the development of resistance and failure of the management program. It is recommended that most toxic insecticides from groups with different modes of action should be used in rotation to prolong the efficacy of insecticides. However, the success of the insecticide rotation scheme depends upon the mode of action, mechanism of development of resistance and cross-resistance potential that are needed to be confirmed in the future to prolong the efficacy of these insecticides.
CONCLUSION
Our investigations showed the variable susceptibility in Ae. aegypti larvae to different insecticides and demonstrates a better understanding about their susceptibility towards traditionally used and newly introduced insecticides. However, variations in insecticide susceptibility may be attributed to a variety of factors; heterogeneity of population, extent of usage and mode of action of insecticides, development of resistance, cross-resistance patterns, etc. which need to be investigated comprehensively to formulate and recommend the measures for insecticide resistance management.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi for providing financial assistance to carry the experiment. The authors extend thanks to Dr. Savithri Singh, Principal Acharya Narendra Dev College for providing laboratory and culture facilities to conduct the experiments.


