All published articles of this journal are available on ScienceDirect.
Repellence Activity of Cymbopogon Citratus (DC) Extracts on Anopheles Mosquitoes using Swiss Albino Rat and Human Volunteer
Abstract
Introduction:
Insect-transmitted disease remains a major source of illness and death worldwide. Anopheles stephensi and Anopheles culicifacies are the important vectors of malaria, malaria continues to be a major public health problem in the tropical world. This study is aimed at carrying out repellence activity of Cymbopogon citratus (Lemon grass) extracts on Anopheles mosquitoes using swiss albino rat and human volunteers.
Methods:
Methanol, chloroform and water were used to extract the bioactive compounds of plant leaves, qualitative and quantitative phytochemical components of plant extracts were determined, twenty-five laboratory-reared 3 day old Anopheles mosquitoes which had been starved for 5 hours were used, extracts and the commercial insecticide N, N-diethyl-3-methylbenzamide (DEET) were applied topically on the skin of uncovered portions of the hand of the human volunteers and abdomen of swiss albino rat after the abdominal hairs has been shaved and the landing rate of mosquitoes were observed to calculate percentage repellency.
Results:
The result revealed that methanol extract had the highest percentage yield of 10.3%, tannin and alkaloid were present in all extracts. DEET had 100% repellency to Anopheles mosquitoes on both human volunteers and rats for 300 minutes post application, in human volunteers, water, chloroform and methanol extracts, has percentage repellency reduced from 100% to 94% after 60 minutes, 100% to 94% after 120 minutes and 100% to 83% after 150 minutes post application respectively while in swiss albino rat, water, chloroform and methanol extracts, percentage repellency reduce from 100% to 87% after 90 minutes, 100% to 87% 150 minutes and 100% to 90% after 180 minutes post application respectively.
Conclusion:
Methanol extracts of C. citratus leaves could be used for the development of topical cream that repels mosquitoes for effective control of malaria.
1. INTRODUCTION
Malaria remains one of the most prevalent diseases in the tropical world. With 200 million to 450 million infections annually worldwide, it causes up to 2.7 million deaths including 1 child every 30 s [1]. Malaria is a mosquito-borne disease is caused by parasitic protozoans (a type of unicellular microorganism) of the genus Plasmodium. Five species of Plasmodium can infect and be transmitted by humans [2]. The vast majority of deaths are caused by P. falciparum, P. vivax, while P. ovale, and P. Malariae [3].
Malaria is common in tropical and subtropical regions because rainfall, warm temperatures, and stagnant waters provide an environment ideal for mosquito larvae. The rainy season may be important in malaria transmission due to high biting populations. Commonly, the disease is transmitted by a bite from an infected female Anopheles mosquito, which introduces the organisms from its saliva into a person’s circulatory system, parasites can also be transmitted by blood transfusions, although this is rare [4].
Prevention of malaria includes medications, mosquito elimination and prevention of mosquito bites [5]. The presence of malaria in an area requires a combination of high human population density, high anopheles mosquito population density and high rates of transmission from humans to mosquitoes and from mosquitoes to humans [6]. If any of these is lowered sufficiently, the parasite will eventually be eliminated from that area. Furthermore, disease transmission can be reduced by preventing mosquito bites by using mosquito nets and insect repellents, or with mosquito-control measures such as spraying insecticides and draining standing water [7].
Control of mosquitoes is something of utmost importance in the present day with rising number of mosquito borne illnesses. Chemical control using synthetic insecticides had been favorable so far because of their speedy action and easy application. Certain plant species containing essential oils have proved efficacy as larvicides, adulticides, ovicides and repellents against different species of mosquitoes [8]. Natural pesticides, especially those derived from plants are more promising in this aspect. Aromatic plants and their essential oils are very important sources of many compounds that are used in different respects. Most of the mosquito control programmed target the larval stage in their breeding sites with larvicides [9]. Personal protective measures including repellents are widely used to prevent the transmission of mosquito-borne diseases by minimizing the contact between humans and vectors [10].
Repellent properties of several leaves extracts appear to be associated with the presence of monoterpenoids and sesquiterpenes [11]. Monoterpenes such as a-pinene, limonene, terpinolene, citronellol, citronellal, camphor and thymol are common constituents of a number of leaves extracts and they have mosquito repellent activity [12, 13]. Cymbopogon citratus (lemon grass) is commonly used in teas, soups and curries. This plant has been widely recognized for its enthnobotanical and medicinal usefulness [14]. Hence this study aimed at studying the repellence activity of Cymbopogon citratus (Lemon grass) extracts on Anopheles mosquitoes using Swiss albino rat and human volunteers.
2. MATERIALS AND METHODS
2.1. Collection of Plant Materials
Leaves of Cymbopogon citratus were collected from South gate area of Federal University of Technology, Akure, Ondo State, Nigeria, into a clean sack. The leaves were well screened and the unwanted ones were removed before processing for extraction.
2.2. Processing and Extraction of Cymbopogon Citratus
The fresh leaves were air dried for four weeks in the absence of contaminations until fully crispy. The leaves were crushed and pounded using clean mortar and pestle and pulverized into fine powder by blender. The powdered leaves were kept in an airtight container to avoid the absorption of moisture. The powdered sample was soaked for 72 hours in 70% methanol, chloroform and aqueous water in the ratio 1:10 each (300g of the powdered sample in 3000 millimeter of 70% methanol, chloroform and water) as solvents to extract the bioactive compounds, after which the samples were first sieved using muslin cloth and filtrate was filtered using membrane filter of 0.45µm pore size. The filtrates were vaporized to dryness using rotary evaporator and were preserved in a sterile bottle at temperature of 4°C for further use [15].
2.3. Phytochemical Screening of the Plants’ Extracts
The qualitative and quantitative phytochemical screening of the extracts were carried out using standard procedure as described by [16, 17].
2.4. Reconstitution of the Methanolic, Chloroform and Water Extracts
The crude extract of methanolic, chloroform and water leaves was reconstituted with 30% dimthylsulphoxide (DMSO) at concentration of 400 mg/ml [18].
2.5. Selection of Anopheles Mosquitoes used in this Study
The Anopheles (Anopheles gambie) mosquitoes were obtained from stagnant water using physical characteristics of the larvae for identification. They were reared and maintained at 27±3°C and 80±10 Relative Humidity (RH) [19]. They were reared on a diet of floating catfish feed. The adults’ mosquitoes were maintained in screened cages on 10% sucrose solution for 24 h. Repellency was tested against 3 day-old blood-starved mosquitoes, and for each test, 25 mosquitoes were used [20].
2.6. Mosquitoes Repellency Experimental Design
Human volunteers used in this study were students of The Federal University of Technology, Akure, Ondo State of Nigeria. The volunteers were educated about this study after seeking their verbal consent. Before the test, the skin of volunteers were washed using unscented soap and the 5.0 ml of extract being tested was applied to the exposed part of the skin. After the application, the hand was not allowed to be rubbed, touched, or wetted. An untreated hand was used as control (Negative control) and the one with DEET was used as a standard (Positive control).
Volunteers were exposed for 5 hours (from 5pm to 10pm) to mosquito bites. Bright torchlights were used to view the volunteers’ reaction to bites. The observations were recorded at 30 minutes intervals. All experiments were run at ambient temperature (27 ± 2 oC) and relative humidity of 80 ± 10%. The numbers of mosquitoes landing on hand were recorded and the mean percentage protection was calculated [21]. Three replicates were run for each repellent and in each replicate different volunteer were used to nullify any effect of skin differences on repellency.
2.7. Animal Testing
The extracts were tested on laboratory animals. The animals were laboratory rats with average 350 to 400 g weight. A 4 x 8 cm of animal abdomen hairs was shaved then washed and cleaned with 75% ethanol. Treatments were 5 ml of the extracts containing 20% of active ingredients of extracts, 5ml of DMSO (Dimethyl sulphoxide) as Negative control and 0.5 ml DEET (N, N-diethyl-3-methylbenzamide) in the same way were used as a positive control. After treatment, the animal was transferred into the cage in which the treated position was exposed to mosquitoes for 5 hours. Each test was repeated three times, replacing new mosquitoes and new animal and number of bites through the fabrics was recorded.
For each repellent, mosquitoes which had been starved for 24 hour were used. Percentage repellency was calculated using the method of [22] as illustrated below,
% repellency = [(Nc – Nt) / Nc] x 100
where Nc = is the number of mosquitoes landing on the control subject,
Nt = is the number of mosquitoes landing on the treated subject used in each of the experiments.
2.8. Ethical Considerations
Ethical permit for human volunteer assay was granted by the ethical review committee of the Federal University of Technology, Akure, Ondo State of Nigeria while the ethical permit for animal assay was granted by the animal ethical review committee, Department of Animal Production and Health, Federal University of Technology, Akure, Ondo State of Nigeria.
3. RESULTS
3.1. Percentage Yield of Plant Extracts
Table 1 shows the percentage yield of plant extracts. The methanol extract of C. citratus had the highest percentage yield of 10.3% while the least percentage yield was observed in the water extracts 7.2%.
| Solvent | Original weight(g) | Extracted weight(g) | % Yield |
|---|---|---|---|
| Methanol | 200 | 20.6 | 10.3 |
| Chloroform | 200 | 18.7 | 9.35 |
| Water | 200 | 14.4 | 7.2 |
3.2. Qualitative Phytochemical Result of C. Citratus Extracts
The result of the qualitative phytochemical analysis of C. citratus extracts is showed in Table 2. The result review the presence of tannin and alkaloid in methanol, water and chloroform extract, however, steroid and anthraquinone were absent. Water extract of plant showed the presence of tannin, alkaloids, terpenoid, flavonoid, saponin and glycosides, while chloroform extract review the presence of tannin and alkaloid. Also, methanol extract and chloroform extract showed the presence of tannin, alkaloids, terpenoid and saponin.
| Phytochemical | CCA | CCC | CCM |
|---|---|---|---|
| Tanin | + | + | + |
| Alkaloid | + | + | + |
| Terpenoid | + | - | + |
| Flavonoids | + | - | - |
| Saponin | + | - | + |
| Glycosides | + | - | - |
| Steroid | - | - | - |
| Anthraquinone | - | - | - |
3.3. Quantitative Phytochemical Result of C. Citratus Extracts
The quantitative phytochemical result showed that methanol extract has high total phenol composition of 43.81±0.15 ppm, this was closely followed by the chloroform extract. Water and methanol extract of C. citratus had higher composition of terpenoid with water having 25.25±0.08 ppm and the methanol extract having 41.03±0.00 ppm.
This is shown in Figs. (1 and 2). The amount of terpenoid in chloroform extract was minute. The phytate content was also found to be higher in the chloroform extracts. The least phytochemical in all the extracts was steroid and glycosides both of which were at minute levels.
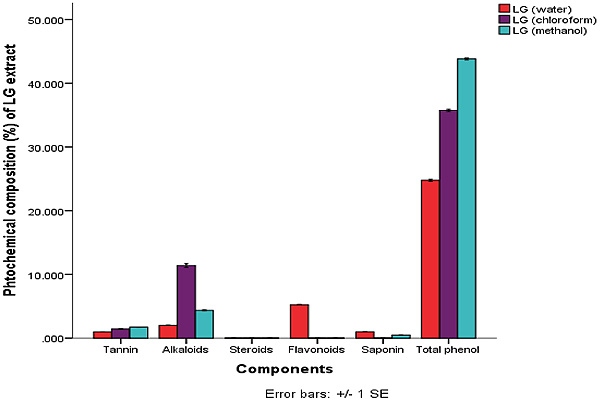
Legends: LG (Water) = Lemon Grass Water, LG (Chloroform) = Lemon Grass Chloroform, LG Methanol = Lemon Grass Methanol.
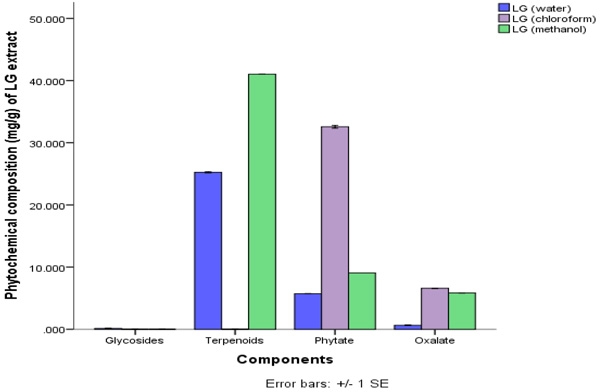
Legends: LG (Water) = Lemon Grass Water, LG (Chloroform) = Lemon Grass Chloroform, LG Methanol = Lemon Grass Methanol
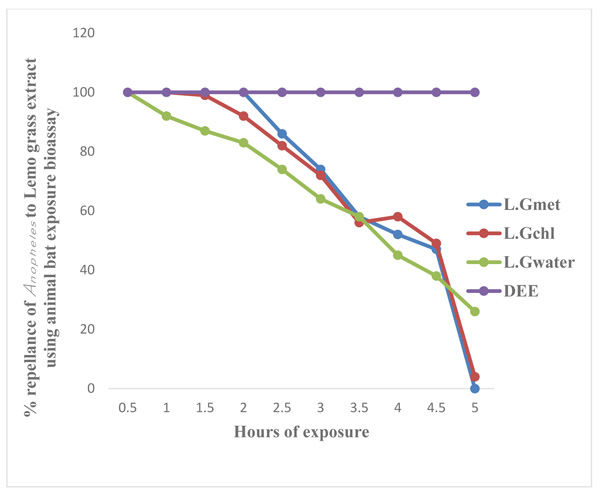
Legends: LG Met = Lemon Grass Methanol extract, LG Chl = Lemon Grass Chloroform extract, LG Water = Lemon Grass Water extract, DEET = Diethyltoluamide
3.4. Repellent Properties of Methanol, Chloroform and Water Extract of C. citratus
Figs. (3 and 4) show the results of laboratory study on animals and human volunteer comparing the repellent properties of methanol, Chloroform and water extract of C. citratus, with the commercial insecticide (DEET) (positive control) against Anopheles mosquitoes. The result shown in Fig. (3) using experimental rat revealed that DEET had 100 percent (%) repellency to Anopheles mosquitoes at 0.5 (30 minutes) to 5 hours, also the percentage repellency of methanol, water and chloroform extract reduces with time, water extract repellency reduce from 100% to 94% after 1 hour post application, percentage repellency of chloroform extract reduce from 100% to 94% after 2 hours post application and percentage repellency of methanol extract reduce from 100% to 83% after 2½ hours (2.5) post application. It also revealed that percentage repellency of methanol and chloroform extracts were reduced to 0% after 5 hours post application. Fig. (4) revealed the percentage repellency of Anopheles mosquitoes exposed to C. citratus extract using human volunteers. The result showed that DEET has 100% repellency at 0.5 (30 minutes) to 5 hours. However, percentage repellency of water extract reduced from 100% to 87% after 1½ hour post application, percentage repellency of chloroform reduced from 100% to 87% after 2½ hours post application while % percentage repellency of methanol extract reduced from 100% to 90% after 3 hours post application.
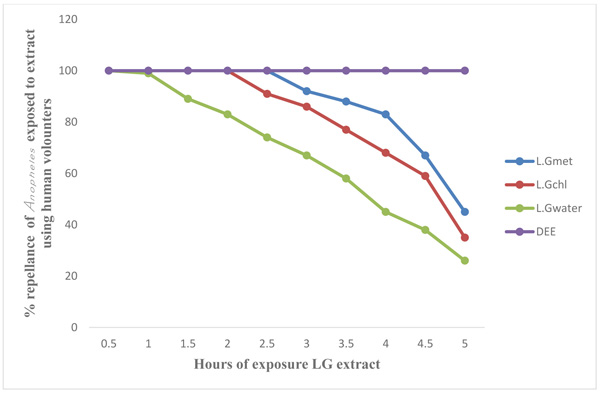
Legends: LG Met=Lemon Grass Methanol extract, LG Chl=Lemon Grass Chloroform extract, LG Water=Lemon Grass Water extract, DEET=Diethyltoluamide
4. DISCUSSION
The percentage yield of the extract from the solvent extraction showed that methanol solvent gave the highest yield. This may be due to the fact that methanol is a polar solvent and may be able to extract the active ingredients of the plant without denaturing them. This agreed with the findings of Iloki et al., [23], who reported that methanolic extracts of some certain plants contained the highest total amounts of phenolics, flavonoids and condensed tannin compounds when compared to percentage yields of the other solvent systems: (chloroform and water). The presence of phytochemicals is an indication that the plants contain bioactive component that might be responsible for the antibacterial and repellency properties of the extracts. This result is in accordance with the report of Soares et al., [24], who reported the presence of some phytochemical constituents in these plants.
Moreover, depending on the type and amount of alkaloids present in the methanolic extract, this could present some kind of toxicity that should be characterized and monitored, if necessary [24]. Total phenols are secondary metabolites found in high amount in extracts with a very low content of tannin and saponin. This agreed with the work of Iloki et al., [23], who reported high total phenol in C. citratus leave extract. Alkaloids are nitrogenous compounds that showed insecticidal properties at low concentration and are often toxic to vertebrates. Tepenoids are among the most widespread, structurally diverse plant produced natural pesticides. A triterpinoid obtained from the Neem tree and several monoterpenes such as citronella, pinene, linolool from essential oils are common terpenoids known to possess insecticidal activities. Generally, the monoterpenoids and sesquiterpenes are associated with repellent properties of several essential oils [25].
Methanol extract showed better repellent properties than chloroform and water extract. One major observation of the repellent properties of C. citratus is that it induces skin irritation /skin rash on the body of the shaved albino rat and the fore arm of the human volunteers. This observation agreed with the findings of Shine et al., [26], who reported on the skin irritation potential of C. citratus extract. Motoyshi et al., and Christopher et al., [27, 28], have also reported that citral, a major component of the C. citratus can induce skin irritation in human.
The result of the repellence potential of the extracts obtained in this study revealed that C. citratus extracts were 100% effective for animal bait and human volunteers for at least 2 hours post application, after which repellence fell slowly from 100%. This is in accordance with the work of Oshaghi [22], which stated that 100% repellence of plant-derived repellants rarely exceeds 2 hours. He also stated that plant repellence above 60% will be considered good and that below 60% will be considered poor. Amer and Melhorn [29] reported that the repellency activity of the plant extracts against mosquitoes could be due to the presence of some phytochemical constituents in the plants. Although, repellent efficacy of plant extract are generally attributed to some particular compounds but if a synergistic phenomenon established among these metabolites, then it may also result in an increased bioactivity compared to isolated components [30]. However; Adeniran and Fabiyi [14] reported that the pure lemongrass oil provided 95% repellency for three (3) hours. The extended repellency efficacy reported by Adeniran and Fabiyi [14], compared to this study could be as a result of different repellency efficacy of essential oil and leaf extract because the main phytochemicals responsible for repellency may be concentrated in oil than leaf, also, the oil used has been purified which could also enhance the higher repellent efficacy reported.
This study also showed that the application of C. citratus repellent compounds gave acceptable percentage biting protection against Anopheles mosquitoes which is not significantly different (P≤0.05) from the protection seen in the commercial insecticides (DEET) used as positive control under similar conditions.
This study has shown that leaf extracts of C. citratus possess repellent properties against Anopheles mosquitoes. The vast and abundant phytochemical content of the extracts should be harnessed for developing and producing novel biological repellents.
CONCLUSION
The presence of bioactive component of the leaf extract suggest that this plants are physiologically active, supporting the claim by traditional healers. Isolation, identification and purification of these phytochemicals and determination of their respective potencies and toxicological evaluation with the view to formulating antibiotics should be the future direction for investigation.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical permit for human volunteer assay was granted by the ethical review committee of the Federal University of Technology, Akure, Ondo State of Nigeria while the ethical permit for animal assay was granted by the animal ethical review committee, Department of Animal Production and Health, Federal University of Technology, Akure, Ondo State of Nigeria.
HUMAN AND ANIMAL RIGHTS
All the reported experiments on humans in the study were in accordance with Parra JL, Paye m, EEMCO Group (2003) EEMCO guidance for the in vivo assessment of skin surface PH. Skin Pharmacol Appl Skin Physiol 16:188-202. And all experimentation on animals were in accordance with the Association for the Study of Animal Behavior; Animal Behavior Society 2006. Guidelines for the treatment of animals in behavioral research and teaching. Animal Behaviour, 71: 245-53.
CONSENT FOR PUBLICATION
Written and informed consent was obtained for the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


