Hydatid Recurrence Medically Treated by Albendazole
Abstract
Introduction:
Hydatidosis or Cystic Echinococcosis (CE) is a highly endemic parasitosis in Tunisia. The combination of surgery with an adjuvant anti-hydatid medical treatment was shown to reduce the risk of relapses, recurrences and post-operative complications.
Case Report:
We report the case of a liver hydatid cyst recurrence in a woman hospitalized for acute cholangitis of hydatid origin. The patient had a resection of the protruding dome and a bipolar drainage of the main bile duct and cystic cavity with a postoperative biliary fistula and a collection of the residual cavity that required endoscopic sphincterotomy. The patient was then followed up every 6 months. Three years after the intervention, CT scan showed a hydatid recurrence of two new liver cysts. Surgery was expected to be difficult and risky. Medical treatment with albendazole was decided before surgery.
The CT scan performed after 9 months showed important regression of two hydatic cysts. Medical treatment has been extended. Two years later, CT scan was in favor of an involuted aspect of both cysts.
The treatment of CE is primarily surgical; medical treatment alone or associated with surgery can be a good alternative, especially in case of hydatid recurrence and when surgery is risky, mainly in stage I hydatic cyst.
1. INTRODUCTION
Cystic Echinococcosis (CE) is a worldwide zoonotic infection caused by the larval stage of cestode, Echinococcus (E.) granulosus [1-6]. In fact, there are 9 species of Echinococcus: E. vogeli, E. oligarthus, E. multilocularis (responsible for alveolar echinococcosis), E. shiquicus, E. felidis, E. granulosus sens stricto, E. equinus, E. ortleppi and E. canadensis [1].The most common species in the world is E. granulosus and this exists exclusively in Tunisia [6]. The parasite cycle takes place between the definitive host (dog) and intermediate host (domestic animals), especially sheep. Humans are accidental intermediate hosts. Humans become infected by the ingestion of E. granulosus eggs shed in the feces of the infected definitive host, resulting in cystic echinococcosis.
CE has a major impact on public health with serious socio-economic consequences. In humans, CE presents usually symptoms associated with the presence of cysts in the liver (the most frequently affected organ), lungs or other viscera [3-5].
In Tunisia, CE is endemic and considered as a major public health problem, especially in rural areas [6-8].The diagnosis of CE is generally made by a combination of imaging and serology. The main treatment modalities are surgery and medical therapy. Each of these modalities has its limitations.
Although surgery remains the most common approach in the treatment of CE, its major disadvantage is the recurrence or the incomplete destruction of the cyst [2, 4]. In the early 1970s, albendazole was found to be effective against E. granulosus and has since been used by many researchers in the treatment of CE [1, 9-11]. However, first-line treatment with this molecule is not approved by some experts who considered it with limited efficacy [3].
We report herein a case of hydatid recurrence medically treated by albendazole.
1.1. Case Observation
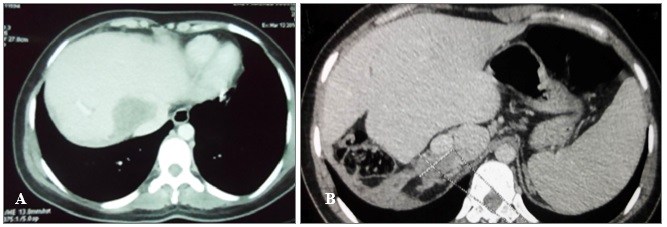
Our case concerns a 26-years-old single woman with no children originating from Sbitla (Governorate of Kasserine, center of Tunisia) who was admitted to the emergency department of Farhat Hached University Hospital of Sousse for acute cholangitis. The patient had no previous pathological history. The abdominal scan performed on the same day showed a giant type III (multivesicular) hepatic cyst of the hepatic dome measuring 25 cm in diameter (Fig. 1). The CT appearance of liver lesions is typical of the hydatid cyst of the liver due to epidemic Echinococcusgranulosus in Tunisia.

The patient was operated on prominent dome resection, and a bipolar drainage of the main bile duct and of the cystic cavity was performed. The postoperative follow-up revealed a persistent biliary fistula that was responsible for a collection within the residual cavity, which necessitated an endoscopic sphincterotomy.
Then, the patient was followed-up by ultrasound every 6 months.
The ultrasound and CT scan control carried out at 2 years and 7 months after the surgical intervention showed a disease relapse by the appearance of 2 type I (univesicular one) new cysts that involved the hepatic dome and the segment I of the liver. The cysts measured 8 and 6 cm, respectively (Fig. 2). In addition, CT scan showed a compression of the hepatic Cavo-hepatic intersection by the cyst of the dome, leading to a Budd-Chiari syndrome. Surgery was expected to be difficult, problematic and risky; and it was decided to first treat the patient medically. Albendazole (Zentel, Z ZOLE, Opalia Pharma, Tunisia) is administered at the dose of one tablet of 400 mg daily for 21 days followed by a discontinuation period of 7 days. Indeed, we usually put patients on albendazole (peri-operatively in case of multiple echinococcosis and / or recidivated) to reduce the risk of hydatic recurrence.

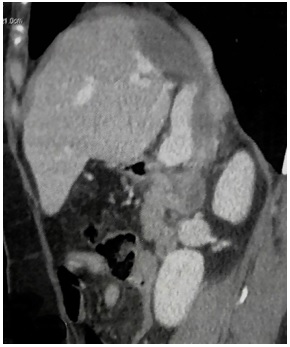
Nine months after medical treatment was initiated, the CT scan control showed an important regression of the size of the cystis’ size (3.7 cm for the cyst of dome and 3 cm for the cyst of segment I) (Fig. 3). In addition, the two cysts showed a collapse appearance with a dense content.
Albendazole was considered to have a positive effect and was continued at the same regimen.
At the end of the 24th month of medical treatment (February 2015), CT scan control showed the hydatid cyst of the dome to be unchanged and a slight decrease in the segment I cyst’ size (2.8 cm) (Fig. 4).
According to these findings, the effect of medical treatment was considered satisfactory and additional surgical treatment was no more needed. Medical treatment was stopped on 12 March 2015.
It is important to note that no side effect was registered during the whole course of treatment. The patient did not receive any other treatment during the study period. She was not hospitalized either. Indeed, the patient had a regular liver check every 3 months during her treatment without any toxic effect recorded.
On the other hand, serological testing by ELISA (Ridascreen®EchinococcusIgG) was positive all along the course of the disease. This test ELISA detects simultaneously antibodies against E. granulosus and E. mtilocularis.
2. DISCUSSION
In Tunisia, hydatidosis is a major public health concern. Its prevalence in humans as revealed by epidemiological, morphological and serological surveys ranges between 1 and 5% according to regions. The annual surgical incidence rate is estimated at 12.6.p. 100.000 inhabitants and exceeds 40.p.100.000 in the governorate of Kairouan (central of Tunisia) [6, 7].
In Tunisia, Hyper-endemic areas are mainly located in the center and the North-West [6, 12].
The sensation of heaviness of the right hypochondrium is the most frequent manifestation. In case of cyst complicated by biliary fistula or infection, fever and jaundice are often observed. Asymptomatic forms are not uncommon
Surgery remains the most common approach in the treatment of CE with however an estimated risk of relapse from 2 to 15% [2, 13].
The hospital stay after surgery is often prolonged and can reach up to one month, especially in patients with a hydatic complication of the biliary or bronchial fistula type. One of the problems that can be encountered after surgical treatment of patients with CE is the risk of therapeutic failure due to non-radical surgical procedures or spilling of parasitic material [2, 3].
According to previous studies, even under optimal ideal conditions, the postoperative mortality rate ranges between 0.9% and 3.6% for the first operation, with a considerable additional morbidity. The risk increases with additional surgical interventions (6% for the second operation and 20% for the third one) [2, 14]. Hence, close post-surgical monitoring is highly required.
Albendazole is an anthelmintic drug of the benzimidazole class. In addition to its activity on many helminths [15], it is currently recommended in the medical treatment of CE [2, 3].
Albendazole blocks the polymerization of the cytoskeleton tubulins and the absorption of glucose by the parasite. In addition, it decreases the production of Adenosine Triphosphate (ATP) causing energy depletion and hence the immobilization of the cestode [2]. In Tunisia, albendazole is the most widely used antiparasitic drug, prescribed in various parasitic infections, including hydatidosis.
In our patient, albendazole showed a very favorable effect as reflected by the change in the appearance and the reduction of the size of both hydatid cysts.
The natural history of liver hydatid cysts is well known. A type I cyst progresses in several years and does not regress spontaneously. This allows us to conclude that remission was achieved with albendazole.
Our findings are in accordance with many previous studies that have shown that long-term treatment with albendazole, either prescribed alone or in combination with surgery, can be very effective in a high proportion of patients [2, 16]. In addition, other studies have shown that treatment with albendazole can reduce the risk of hydatid recurrence. This efficacy has been demonstrated in about 70% of cases [17-19]. Some authors are not convinced of the interest of albendazole in monotherapy. However, its interest in association with the surgery is now acknowledged in case of cyst type I, II and III [3].
According to the WHO recommendations, medical treatment is indicated in inoperable primary liver CE, in pulmonary CE, in patients with multiple cysts affecting two or more organs and peritoneal cysts [20, 21].
Our patient was treated according to the cyclical protocol (10-12 mg/kg in 2 divided days, per 30 days separated by 15 days) [22]. Recent data showed that continuous treatment over 3 months or more, could improve the efficiency of albendazole without additional toxicity [2, 3].
A prolonged follow-up period for albendazole-treated patients is essential before a definitive assessment of the therapy effectiveness can be made.
In our patient, no toxicological symptoms attributable to albendazole were detected over the 2-years treatment period. The hepatic toxicity of albendazole is well known and can be prevented by temporary discontinuation. On the other hand, long-term medical treatment or perioperative treatment with antiparasitic drugs would be costly for health insurance funds if prescribed to all patients
Conventional tools used for monitoring patients with CE include imaging techniques such as ultrasound or CT and serological testing [19, 23]. However, serological testing is not of high significance in the evaluation of treatment efficiency as the decrease in antibody titers was delayed by continuous antigen stimulation due to the persistence of parasitic biomass in deal cysts [3].
The immunoblot based on the detection of P29 protein was shown to be much more valuable in the post-therapeutic follow-up of patients [24].The disappearance of this band is considered as a healing criteria.
CONCLUSION
This case demonstrates that remission with prolonged treatment with albendazole is possible. It also indicates that antiparasitic drugs could be an alternative to surgery for young hydatid cyst (type I, II), recurrence, multiple localization and/or in case of difficult planned surgery.
Medical treatment with this drug can be used as an alternative to surgery when the hydatic cyst is younger, surgery is risky and post-operative complications are expected.
We recommend that before scheduling a difficult surgery or requiring major liver resection, to extend the duration of medical treatment beyond 3 months and follow up by imaging control. A good outcome can prompt to offer an exclusive medical treatment.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the University Hospital Farhat Hached Sousse, Tunisia and was conducted in compliance with the guidelines (OMB No. 0990-0279).
HUMAN AND ANIMAL RIGHTS
No animals were used in the experiments. All the reported experiments involving humans used in the study were in accordance with the ethical standards of the committee responsible for human experimentation and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Written and informed consent was obtained from all the patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared None.


