Parasitological Quality Evaluation of Pipe Waters in Abidjan, the Capital City of Cote D’ivoire
Abstract
Background:
Treatment and wastewater disposal lack in Abidjan leads to wastewater discharge in the natural environment without treatment. These waters were loaded with pathogenic microorganisms that are the causes of many diseases.
Objectives:
This study aims to assess the parasitological quality of water from pipes of Abididjan City, studying protozoa and helminths.
Methodology:
400 samples were collected in three municipalities and 19 sites. Parasites were identified using sedimentation.
Results:
Biological analyzes revealed 269 (67.2%) positive samples, with the presence of 19 taxa belonging to helminths (nematodes, trematodes) and protozoa group (amoeba, ciliates, flagellates). Protozoa are most common with a clear dominance of amoebas class followed by ciliates and flagellates. In 269 positives samples, Entamoeba coli (160 (59.48%)), Endolimax nana (112 (41.64%)), Giardia spp (71 (26.4%)), and Paramecium caudatum (66 (24.54%)) are the most common species. The spatial distribution showed that Yopougon commune was the most parasitized, followed by Abobo-Adjame.
The most parasitized sites were CHU, SEL, and AG. At the seasonal level, the dry season was where the parasite load was highest regardless of municipality and sites.
Conclusion:
The presence of these parasite groups and the diversity of parasitic taxonomic indicates faecal contamination of piped water in Abidjan city. These parasites present a potential danger for these piped waters' direct and indirect uses. Therefore, it is necessary to treat this water before any use and before it flows into surface water to avoid these parasites.
1. INTRODUCTION
One of the Millennium's development goals aims to guarantee access to drinking water. Its availability is a key issue for humanity [1]. In addition, access to quality drinking water in sufficient quantity remains an indisputable factor that influences the economic and social development of human communities and their environment [2, 3].
It is estimated that 884 million people do not have access to safe drinking water worldwide. This leads to diseases that cause the death of around two million people, most of whom are young children [1, 4]. In Côte d'Ivoire, the national drinking water coverage rate is 60%. Climate change, galloping population growth and nitrate pollution are causing groundwater depletion in that country [5]. Abidjan, the economic capital home to 20% of the Ivorian population, has a daily drinking water deficit of 150,000 m3, corresponding to 1.5 million people who do not have access to water [2]. To solve the difficulties of supplying drinking water, the government has recourse to surface waters, from which lagoon waters are real alternatives. However, like the big cities of developing countries, the city of Abidjan does not have an effective water treatment system. The sanitation system is insufficient; only 30% of the population is connected to it, and more than 25% have no sanitation system equipment [6, 7]. Domestic, hospital and industrial wastewater is discharged into the natural aquatic environment without pre-treatment. Thus, this wastewater contains pathogenic microscopic organisms (bacteria, viruses, protozoa, helminths), which constitute a risk of contamination for surface and underground water. These organisms are responsible for part of the 4 billion cases of diarrhea worldwide and 1.6 million deaths [8]. From 1991 to 2008, 11% of waterborne outbreaks were caused by parasites [9, 10].
Among these pathogens contaminating water, protozoa and helminths occupy a prominent place [11, 12]. They cause waterborne disease due to protozoa (protozooses) and helminths (helminthiasis). In the aquatic environment, these parasites are found in the form of cysts, oocysts, vegetative forms, eggs and larvae which ensure both their dissemination and resistance in the external environment [12, 13].
These parasitoses are contracted by ingesting water and food containing cysts, oocysts, vegetative forms, eggs of parasites or by direct contact with infesting larvae [13, 14].
Infection with these parasites negatively impacts the health of children, pregnant women, and worker productivity and constitutes a socio-economic burden for developing countries [15, 16]. The prevalence of these parasites in pregnant women in Abidjan City is 54.5% [17]. This high prevalence among these women is due to faecal peril caused by the failure of the sewage disposal systems. Scare data are available on the distribution of protozoa and helminths in sewer water in Abidjan. The only information on these parasites is that of Cissé et al. [6] in the Gourou bassin (Abobo – Adjame – Cocody), where helminths were identified. Yapo et al. [18] also studied the infection risks related to the use of wastewater in Abidjan. These studies were limited to the research on helminths or the assessment of microbial risks associated with the use of wastewater. Regarding the paucity of data on parasites in wastewater, special attention is needed on detecting waterborne parasites to prevent potential outbreaks of parasitic infections. This study aimed to evaluate the parasitological quality of the pipe water of Abidjan by studying protozoa and helminths to collect information on the level of infestation of human and animal populations.
2. MATERIAL AND METHODS
2.1. Study Site
The study was conducted in Abidjan, the economic capital of Côte d'Ivoire, which is located between 5°12' and 5°30' North latitudes and 3°48' and 4°12' West longitudes. It is bordered to the south by the “Ebrié” lagoon, which covers approximately 16% of its area and the Atlantic Ocean. Since 1978, it has included ten communes (Abobo, Adjamé, Attécoubé, Cocody, Koumassi, Marcory, Plateau, Port-Bouët, Treichville, Yopougon) [19], and its population was estimated to be 4.4 million inhabitants in 2014 [20].
2.2. Sample Collection
From July 2019 to September 2020, we collected wastewater samples in different locations throughout the city of Abidjan during the dry and rainy seasons. The samples were collected in different locations in the communes of Yopougon, Abobo-Adjamé and Marcory-Koumassi (Fig. 1).
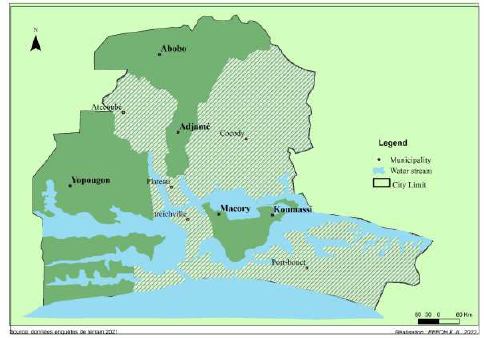
The area selected were those whose wastewater channels have outlets on lagoons. This includes Yopougon: (Carrefour CHU (CHU), Andokoi (AND), Kimi (KIM), Phenix (PH), Marché gouro (MG), Selmer (SEL), Koweït (KW), Sideci (SD); Abobo-Adjamé: Agban (AG), Carrefour II-plateaux (IIP), Carrefour Zoo (ZOO), Corniche (COR), Fraternité-matin (FRM)); Marcory-Koumassi: Sicogi (SIC), Canal (CAN), Zoé bruno (ZB), Pergolat (PER), Saco (SAC) and Amoré (AM).
These different sites are the routine sites of the Chemistry and Environmental Microbiology Unit of the Institut Pasteur Côte d'Ivoire (IPCI). For the samples, an outing was made every Monday in a chosen municipality, and each municipality's different districts were crisscrossed. In each district, sampling zones were selected. Samples were collected from each area using a scoop, and 1.5L of water was taken and then placed in sterile 1.5L containers.
2.3. Laboratory Analysis
Microscopic analysis of the samples collected was conducted at the Parasitology Unit of IPCI in Côte d'Ivoire in Abidjan. Three methods were used to identify parasites:
1. The sedimentation method - consisted of observing the samples as they were taken. It allowed observing the vegetative forms of all kinds of microorganisms in the samples. It was done by observing, with 10X and 40X objectives, the pellets of the samples which had previously undergone sedimentation for 24 hours.
2. The simple concentration method made it possible to concentrate the microorganisms in the different samples. It consisted of reading concentration pellets with 10X and 40X objectives. These concentration pellets were obtained by centrifugation at 4000 revolutions per minute (4000 rpm) for 15 min of the sedimentation pellets.
2.4. Data Analysis
Data were analyzed using GraphPad Prism 5 and Rstudio software. First, a Chi-square test was performed to compare parasite groups, the prevalence of positive samples and parasites by season. A proportion test was performed to compare parasite classes, parasite species and prevalence of parasites according to municipalities and sampling sites. An ANOVA was carried out to compare the prevalence of parasites observed: by municipality according to species, by seasons according to the municipality, by seasons according to sampling sites and finally by seasons according to species. Differences are considered significant when P< 0.05.
3. RESULTS
Observation of the samples collected revealed the presence of helminths and protozoa. Five classes of parasites have been identified in these parasites. Protozoa are of the amoebas, ciliates and flagellates class, and the helminths are of the nematode and trematode class.
3.1. Taxonomic Diversity of Parasites
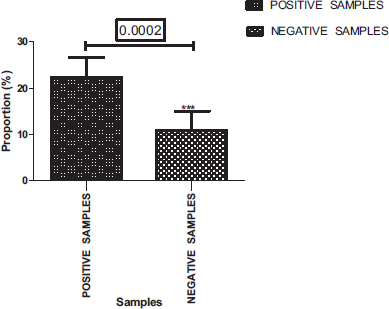
Of a total of 400 samples analyzed, 269 were positives (67.25%) (Fig. 2). Among these positive samples, 548 parasites in different forms, including eggs, larvae and adults of helminths, cysts and vegetative forms of protozoa were detected.
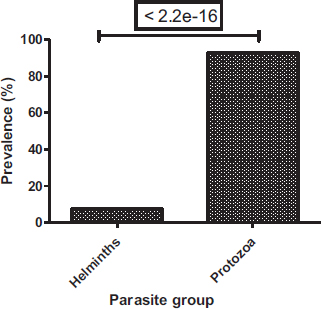
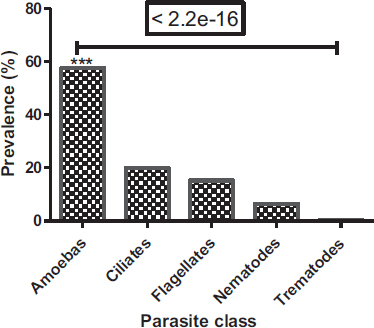
Among the parasites identified, protozoa were common (92.7%), whereas helminths were the least represented (7.3%) (Fig. 3). In protozoa and helminths, five classes have been identified, these are the class of amoebas (316 (57.66%)), ciliates (109 (19.89%)), flagellates (84 (15.33%)), nematodes (36 (6.57%)) and trematodes (3 (0.55%)) (Fig. 4). Amoebas class represents more than half of parasites observed, followed by ciliates and then flagellates. There is a significant difference between the prevalence of protozoa and helminths (Chi-square test: P < 2.2e-16) and a significant difference between parasite classes (Proportion test: P< 2.2e-16).
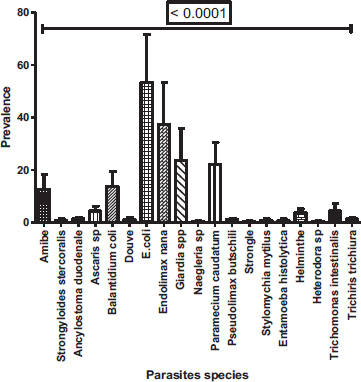
In these 269 positive samples, nineteen parasites species were observed with a prevalence varying from 0.37 to 59.48% (Fig. 5). Among nineteen species, Entamoeba coli (59.47%) was the most prevalent followed by Endolimax nana (41.64%), Giardia sp. (26.4%) and Paramecium caudatum (24.54%). With a prevalence not reaching 1%, Entamoeba histolytica/Entamoeba dispar (0.37%), Naegleria sp (0.37%), Stylomichia mytilus (0.37%), Strongyloide stercoralis (0.74%), Heterodera sp (0.37%), Strongyle (0.37%) were the least represented. A significant difference between the prevalence of the different species was observed (Proportion test: P< 0.0001) with a clear dominance of Entamoeba coli followed by Endolimax nana, then Giardia sp. and Paramecium caudatum.
3.2. Prevalence and Spatial Distribution
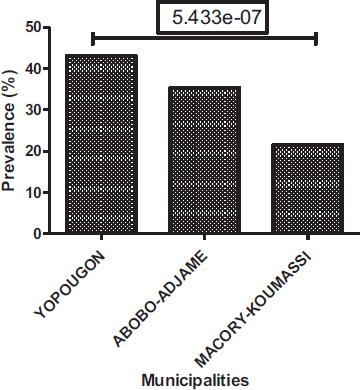
Positive sample prevalence varies from municipality to municipality, with 43.12% in Yopougon, 35.32% in Abobo-Adjame and 21.56% Macory-Koumassi (Fig. 6). Among the three municipalities collected, Yopougon has the highest rate of positivity followed by Abobo-Adjame and Macory-Koumassi. In relative distributions of parasites in the municipalities surveyed, Yopougon has the highest prevalence (48.72%), followed by Abobo-Adjame (35.77%) and Macory-Koumassi (15.51%). Yopougon also has the highest prevalence for helminths (7.42%%) and protozoa (91.82%). The second municipality is Macory-Koumassi (4.46%%) for helminths and Abobo-Adjame for protozoa (70.26%%). Helminths and protozoa were respectively least prevalent in Abobo-Adjame (2.6%) and Macory-Koumassi (27.13%) (Fig. 7). The analysis showed that there is a significant difference between the prevalence of positive sample according to the municipalities (Proportion test: P= 5.433e-7), the prevalence of helminths according to the municipalities (Proportion test: P= 0.0035) and the prevalence of protozoa according to the municipalities (Proportion test: P< 2.2e-16).
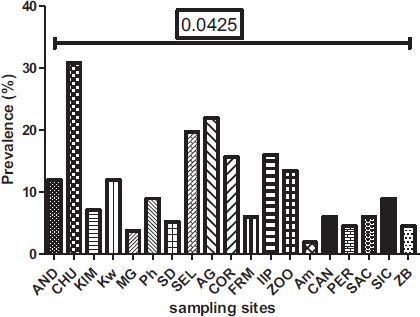
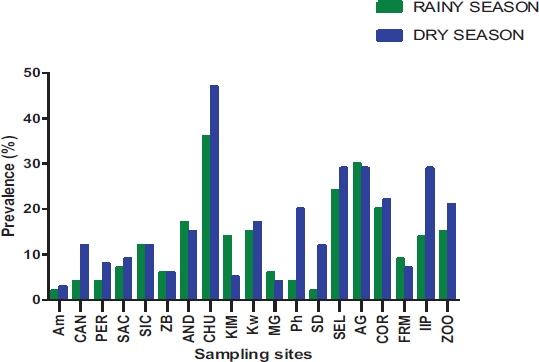
Out of the 19 sites surveyed, at least one parasite was reported, with prevalence varying from 1.86 to 30.86% (Fig. 8). There is a significant difference between the prevalence of parasites according to sampling sites (P= 0.0425) observed. The Yopougon CHU site was where the parasite prevalence was the highest (30.86%).
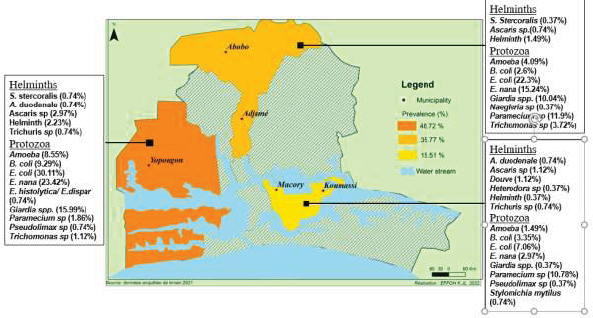
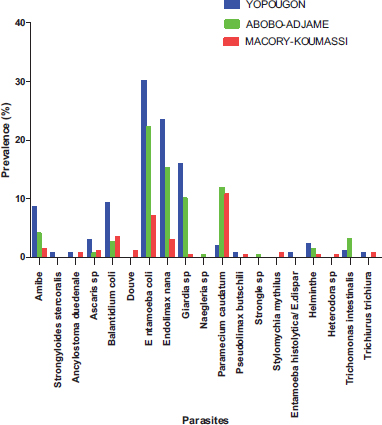
Spatially, the types of parasites identified have a heterogeneous distribution in the municipalities surveyed (Fig. 9). Six parasites (Entamoeba coli, Endolimax nana, Giardia sp, Paramecium caudatum, Balantidium coli and Amoeba) were observed in the all the three municipalities surveyed with Yopougon having the highest prevalence followed by Abobo-Adjame in exception for Paramecium caudatum for which Abobo-Adjame have the highest prevalence (11.9%) followed by Macory-Koumassi (10.78%).
The other parasites were at least observed in one site; among these, Douve (1.12%), Naegleria sp (0.37%), Strongyle (0.37%), and Stylomychia mytilus (0.74%) were observed in a single site in Macory-Koumassi, Abobo-Adjame, Abobo-Adjame and Macory-Koumassi, respectively.
3.3. The Effect of Season on the Prevalence and Spatial Distribution
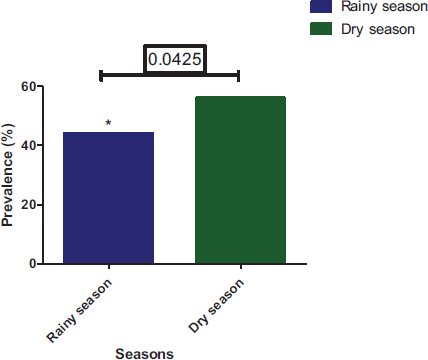
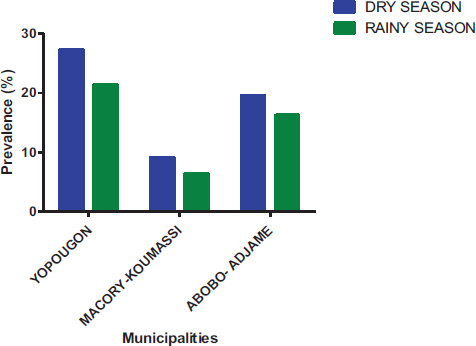
At temporal distribution, parasite prevalence in the dry season was 56.02% and 43.98% during the rainy season (Fig. 10). Parasite prevalence in different municipalities varies according to season. For the dry season, parasite prevalence in Yopougon, Abobo-Adjame, and Macory-Koumassi was 27.37%, 19.53%, and 9.12%, respectively. For the rainy season, parasite prevalence in Yopougon, Abobo-Adjame, and Macory-Koumassi was 21.35%, 16.24% and 6.39%, respectively (Fig. 11). The prevalence of parasites was significantly higher (Chi-square test: P = 0.0425) in the dry season compared to the rainy season. The prevalence of parasites by season according to the municipalities and sampling sites shows that there was a significant difference in the distribution of parasites by season according to the municipality (P=0.011) and the site (P< 0.0001) (Fig. 12). Parasite prevalence in the municipality of Yopougon was relatively higher compared to the commune of Abobo-Adjamé and Macory-Koumassi in the two seasons relative to the sites. AM has the lowest parasite prevalence in all seasons, followed by MG and ZB.
As for the distribution of the types of parasites according to the seasons, there was a significant difference in the occurrence of these parasites between the dry season and the rainy season (P< 0.0001) (Fig. 13). Whatever the season, E. coli cysts followed by E. nana and Giardia sp. then the vegetative forms of P. caudatum followed by B. coli and Amoebae are the most observed parasites.
4. DISCUSSION
The parasitological characterization of sewage water in the city of Abidjan revealed a significant parasitic diversity. A total of 19 species were detected using microscopic observation. These results are consistent with those of Sylla et al. [21], Cissé et al. [6], Raweh et al. [22] and Sylla and Belghyti [23], who respectively detected 13, 10, 10 and 7 species in the lakes of Yamoussoukro (Côte d'Ivoire), in the Gourou collector in Abidjan (Côte d'Ivoire), wastewater of Kenitra (Morocco) and the wastewater of Sidi Yahia in Gharb (Morocco). These parasitic diversities could indicate faecal contamination of these pipe waters because these parasites are excreted in the environment as eggs, larvae, vegetative forms or cysts eliminated with faeces. These results reflect the infestation rate of the human and animal population of the neighborhoods served by this piped water.
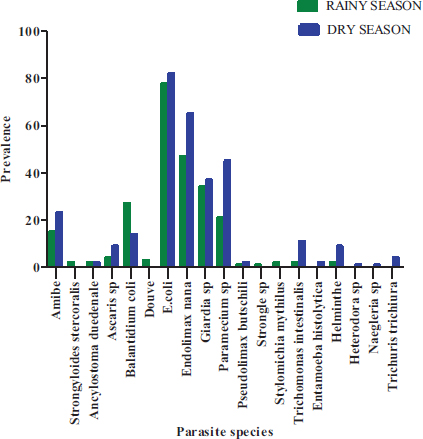
The taxonomic richness found in the three communes of Abidjan is higher than the number of taxa reported in the lakes of Yamoussoukro (13 taxa: Sylla et al. [21]) and the Gourou collector (10 taxa) in Abidjan in Côte d’Ivoire (Cissé et al. [6]). This difference could be explained by the difference in human population density and living in these different localities, the state of the health of these populations and the organic composition of the different sewed waters surveyed.
The taxa were identified to belong to protozoa and helminths of intestinal parasites, observed in the form of eggs, helminth larvae (8 taxa) and cysts, vegetative forms of protozoa (11 taxa). In the helminths, two classes of parasites have been observed: the class of Nematodes (7 taxa) and the class of Trematodes (1 taxon). Concerning protozoa, three classes have been identified: the class of Amoebas (6 taxa), Ciliates (3 taxa) and Flagellates (2 taxa). These different species of parasites observed in these piped waters can reflect the rampant polyparasitism in these surveyed areas. The proportion of parasites in this study is largely composed of protozoa (92.88%), whereas helminths compose only 7.12% of that proportion. These results are different compared to the work of Cissé et al. [6] in the Gourou collector in Abidjan (Côte d'Ivoire) and that of Mbouombouo et al. [12] in surface water in Cameroon, who identified only helminths. Sylla et al. [21] identified 84.6% of helminth and 15.4% of protozoa in Yamoussoukro and Sylla and Belghyti [23]. who identified 14.6% of protozoa, 85.41% of helminths in collector A and 8.7% of protozoa, 91.3% of helminths in collector B in wastewater from Morocco. The high proportion of helminths could explain this difference observed during the rainy season, during which conditions of temperature, humidity, oxygen and solar radiation were favorable to helminth maturation.
However, our results are consistent with those of Ajeagah et al. [24-27] and Mbouombouo et al. [28], who highlighted the abundance of protozoa in waters in Cameroon. This protozoa abundance could reflect faecal contamination of these waters; these microorganisms are released into the environment through faeces. Amoebae are the most observed protozoan class, among which the most recorded species are Entamoeba coli and Endolimax nana. The high prevalence of these species could be explained by the fact that these amoebas are commensal parasites, and their carriage is harmless and goes unnoticed by immunocompetent. In flagellates, Giardia sp., the cysts highlighted in the sewage water samples are consistent with those of Ajeagah et al. [24, 26], who revealed the presence of Giardia sp. cysts in Cameroon waters. These results could be explained by the fact that the cysts of Giardia sp. excreted in the water environment resisted for a long time [29]. Giardia sp. is also one of the main etiological agents of waterborne epidemics worldwide [8, 10]. Among helminths, the class of nematodes has the highest prevalence, and the most observed species is Ascaris sp. These results are consistent with those of Sylla and Belghyti [23]. and Mbouombouo et al. [12]. The dominance of nematodes could be due to their strong resistance to environmental stresses; specifically, the eggs of Ascaris sp. are more resistant to desiccation than other species [30].
The spatial distribution of the parasites varies according to the different municipalities and sampling sites in the city of Abidjan. At the level of the municipalities, Yopougon (48.72%) is the commune where the parasite prevalence is relatively higher compared to the other communes, which are Abobo-Adjamé (35.77%) and Macory-Koumassi (15.51%). At the sampling sites, those of the AM (Macory-Koumassi), MG (Yopougon) and ZB (Macory-Koumassi) with a respective prevalence of 1.86%, 3.72% and 4.46% are relatively the lowest. The sampling sites, those CHU (Yopougon), SEL (Yopougon) and AG (Abobo-Adjame), with a respective prevalence of 30.86%, 19.7% and 21.93%, are relatively the highest. Depending on the municipalities and sampling sites, this variation in parasite abundance could be explained by demographic factors and the size of the sampling site. According to Raweh et al. [22] and EL Guamri et al. [31], the concentration of parasites in wastewater is sensitive to the demographic structure of those connected to the liquid sanitation network. Indeed, the municipalities of Yopougon and Abobo-Adjamé are the largest municipalities of Abidjan, with the highest population density of 1,071,543 and 1,403,636 [20].
At the seasonal level, the prevalence of parasites was higher during the dry season compared to the wet season. These results could be explained by the fact that the water flow is fast in the rainy season, and the suspended matter to which the parasites are attached moves. It could also be explained by the fact that in the dry season, the water observed in wastewater evacuation channels comes from rainwater runoff, latrine products, and domestic wastewater. These results are similar to those reported by Mbouombouo et al. [28] in the Mezan watershed in Bamenda (Northwest region, Cameroon) and Ajeagah et al. [24] in two aquatic biotopes in Yaounde (Cameroon).
CONCLUSION
The microscopic analysis of sewer water of three communes in Abidjan indicates the diversity of parasites, including protozoa which include the class of amoebas, flagellates, ciliates and helminths composed of the class of nematodes and trematodes. These parasites are differently distributed relative to the municipality or the site. This parasitic diversity reflects faecal contamination of human and animal origin.
At the seasonal level, parasitic prevalences have significant differences, with a high prevalence in the dry season because the channels intended to evacuate rainwater are the reception areas for domestic and urban wastewater, therefore whatever the season, they drain water loaded with pathogenic elements and in decomposing organic matter.
This parasitic diversity found in the sewer waters of the city of Abidjan constitutes a health hazard for the populations in contact with these waters and a source of contamination for surface and underground waters. Therefore, it is necessary to treat sewer water before it flows into the environment to avoid enteric diseases caused by these entero-pathogens.
LIST OF ABBREVIATIONS
| CHU | = Carrefour CHU |
| AND | = Andokoi |
| KIM | = Kimi |
| PH | = Phenix |
| MG | = Marché gouro |
| SEL | = Selmer |
| KW | = Koweït |
| SD | = Sideci |
| AG | = Agban |
| IIP | = Carrefour II-plateaux |
| ZOO | = Carrefour Zoo |
| COR | = Corniche |
| FRM | = Fraternité-matin |
| SIC | = Sicogi |
| CAN | = Canal |
| ZB | = Zoé bruno |
| PER | = Pergolat |
| SAC | = Saco |
| AM | = Amoré |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No humans or animals were used for the studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIAL
All the data and supportive information are provided within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


